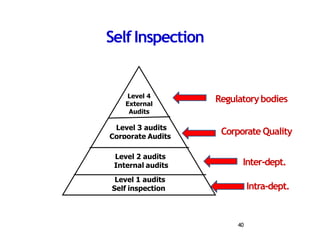
This document provides an overview of Good Laboratory Practice (GLP) from both a regulatory and quality systems perspective. It discusses the scope and importance of GLP for ensuring quality test data, sound laboratory management, and accurate reporting. Key aspects of a GLP-compliant quality system are summarized, including organization, facilities, equipment, documentation, personnel training, sampling methods, and self-inspections. Compliance with GLP is mandated by various regulations to ensure patient safety, meet regulatory expectations, and maintain high customer confidence.