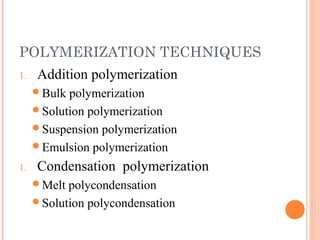
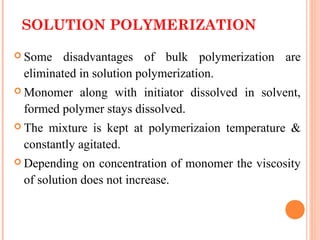
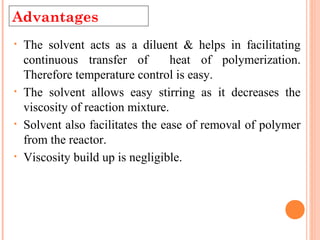
The document outlines various polymerization techniques, focusing on addition and condensation polymerization, with an emphasis on solution polymerization. It discusses the advantages, such as improved temperature control and easier stirring, as well as disadvantages like the need for solvent recovery and lower molecular weight polymers. Additionally, it details the industrial applications and uses of polymers produced through these methods.