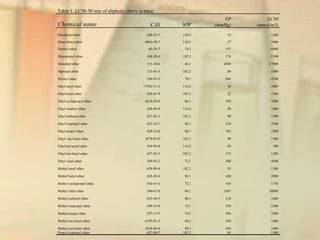
This document outlines the concepts and methodologies of Quantitative Structure-Activity Relationships (QSAR), emphasizing their role in predicting chemical toxicity to address data gaps in risk assessments. It discusses the limitations of traditional testing methods due to high costs and the necessity for alternative approaches, such as QSAR models which derive relationships between chemical structures and biological activity. The document details the steps involved in creating QSAR models and highlights the importance of selecting appropriate biological endpoints and molecular descriptors to ensure reliable predictions.