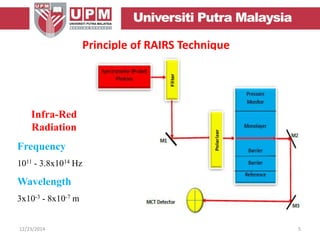
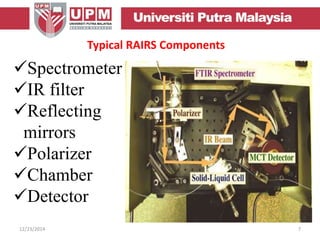
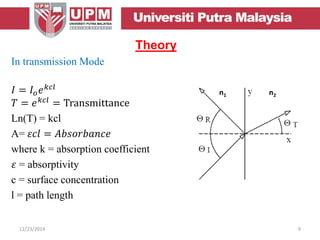
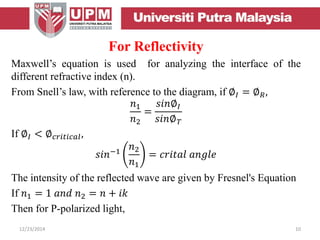
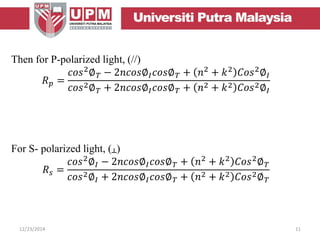
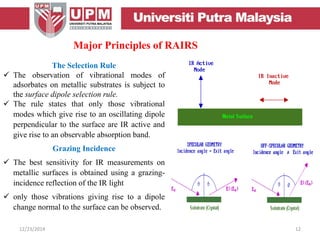
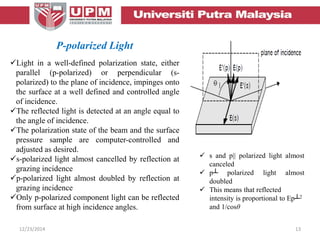
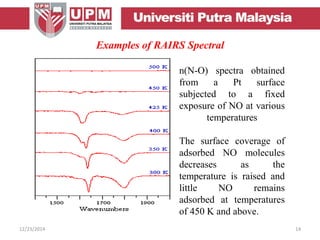
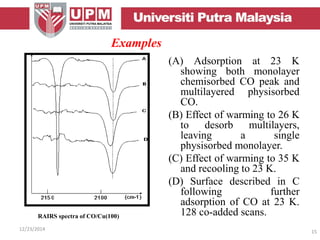
This document provides an overview of vibrational spectroscopy, specifically reflection absorption infrared spectroscopy (RAIRS). It discusses how RAIRS works by directing infrared radiation at a sample surface, analyzing the reflected beam to determine absorbed frequencies. RAIRS has excellent energy resolution and can study surface species and reactions under various conditions. It is most sensitive for observing adsorption of molecules with transition dipoles arranged along the surface normal. The document also covers instrumentation, theory, selection rules, examples of RAIRS analysis, and limitations.