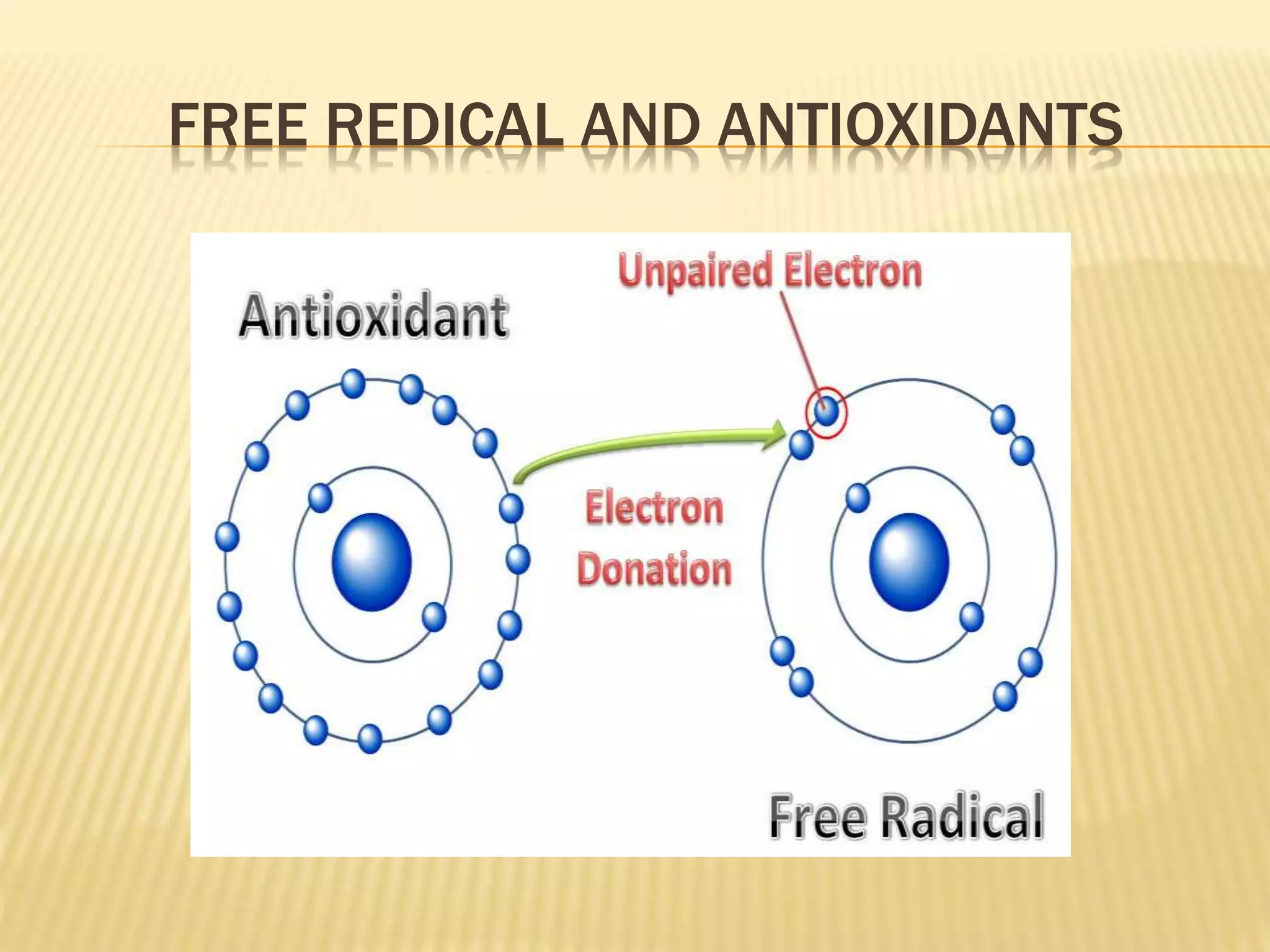
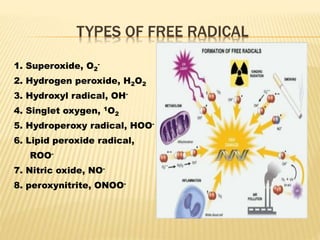
1. Free radicals are unstable molecules that react quickly to gain stability by capturing electrons from other compounds, causing damage. Common types include superoxide and hydroxyl radicals.
2. Free radicals are generated as a normal product of oxygen metabolism in cells and can also come from environmental exposures like pollution, smoking, and radiation. They cause oxidative damage to biomolecules like lipids, proteins, DNA and carbohydrates.
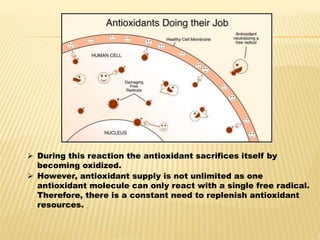
3. This damage is implicated in diseases such as cancer, heart disease, and neurological disorders. Antioxidants help counter the damaging effects of free radicals by reacting with and neutralizing them.