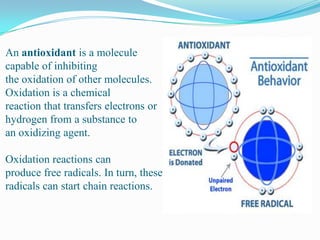
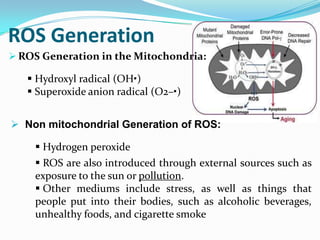
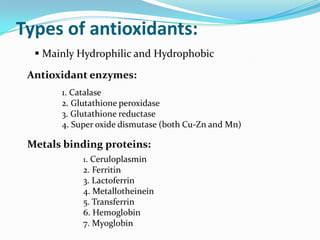
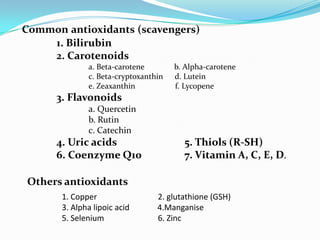
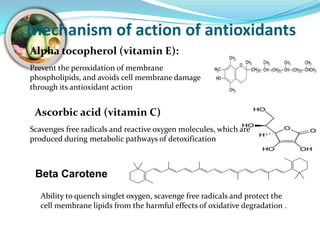
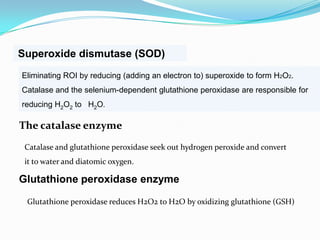
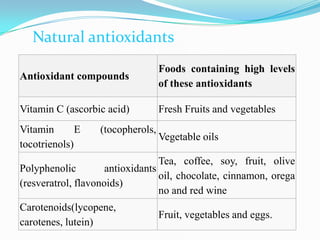
This document discusses antioxidants, which are molecules that inhibit oxidation reactions and protect cells from damage by reactive oxygen species. It describes the main types of antioxidants and free radicals in the body, how antioxidants work to eliminate free radicals, and their importance in preventing disease and aging. Foods contain varying amounts of antioxidants, which must be obtained through diet as the body cannot produce them. Measurement methods are used to determine the antioxidant levels and capacities of foods, juices and other substances.