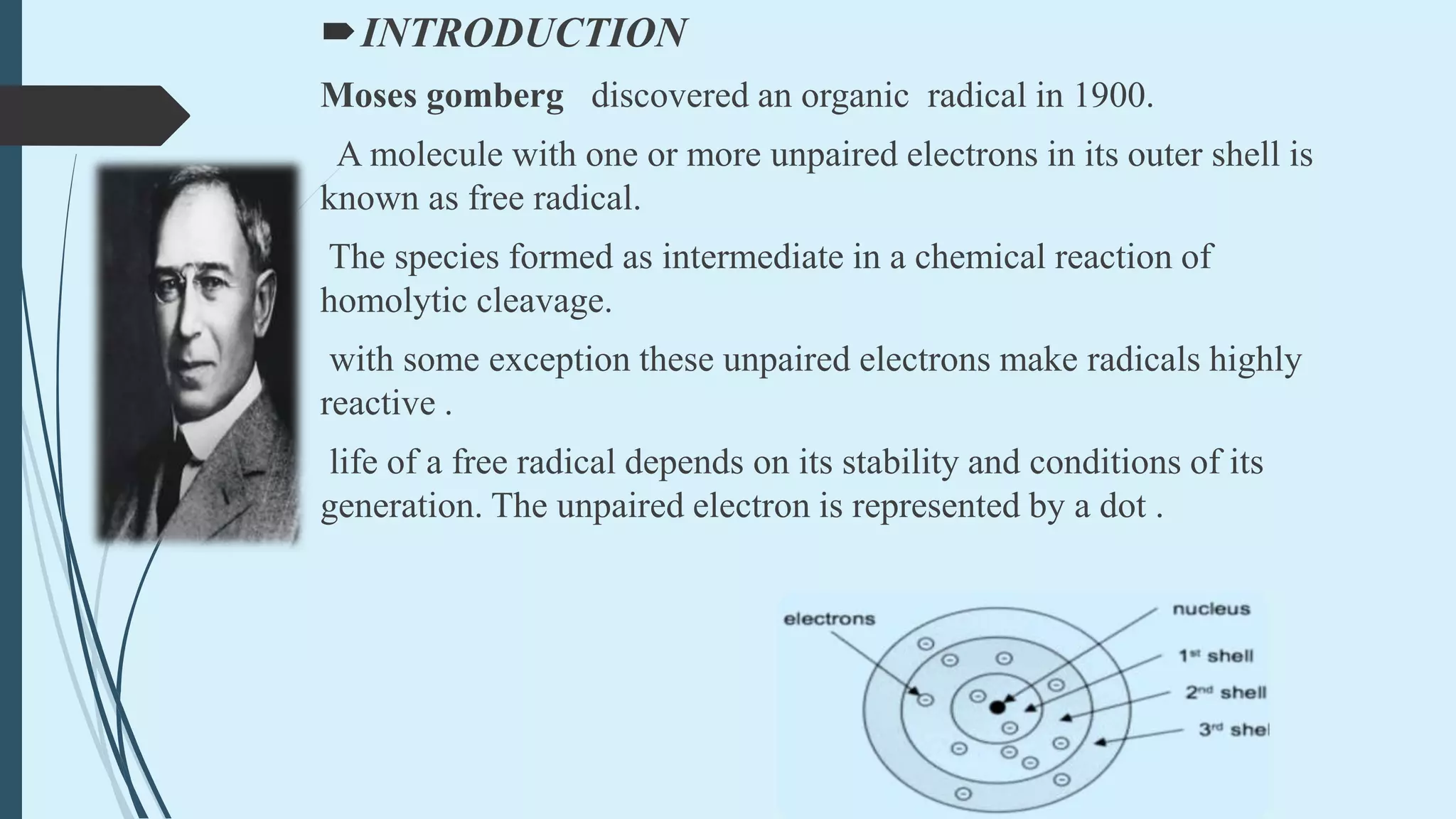
Free radicals are molecules with unpaired electrons that make them highly reactive. They are formed through homolytic cleavage of bonds which leaves each atom with one electron. Common sources of free radicals include mitochondria, xanthine oxidase, peroxisomes, inflammation, and phagocytosis. Free radicals can damage biomolecules like lipids, proteins, carbohydrates, and nucleic acids. This damage is associated with aging, cancer, and acute inflammation. Antioxidants act as neutralizing agents by donating electrons to free radicals and preventing cellular damage. While free radicals can be harmful, they also play beneficial roles in immunity and cellular processes in limited amounts.