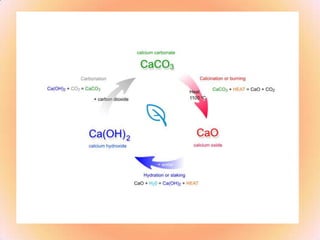
The document lists various calcium compounds including calcium carbonate, calcium oxide, and calcium hydroxide. It describes the conversions between these compounds when heated or reacted with water. The characteristics and uses of each compound are provided, such as calcium carbonate reacting with acids to release carbon dioxide gas and its common uses in antacids, building materials, and cement production.