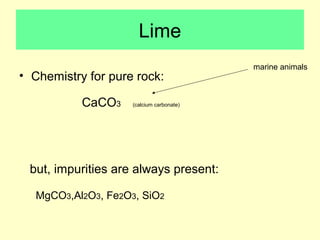
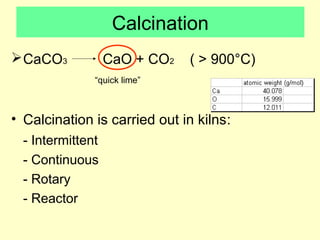
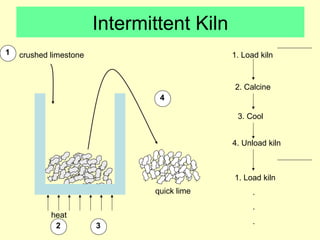
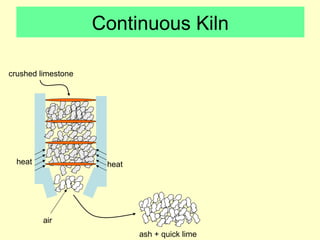
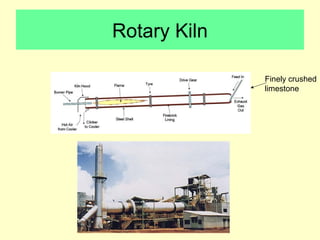
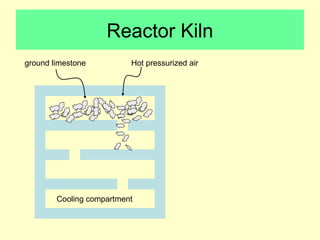
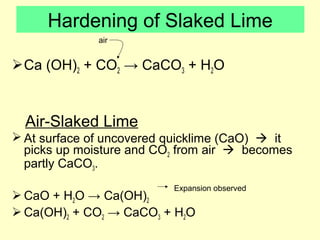
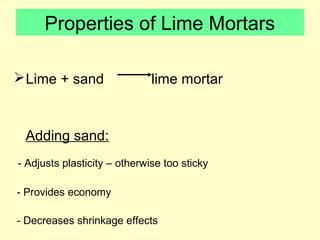
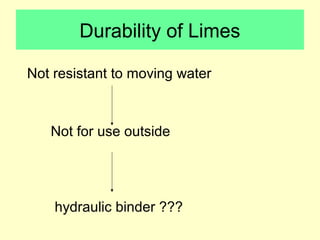
Lime is produced by excavating limestone, crushing it, and calcining it in kilns at high temperatures to produce calcium oxide (quicklime). Quicklime is then slaked by mixing with water to produce calcium hydroxide (slaked lime). Slaked lime hardens over time as it reacts with carbon dioxide in the air. Lime is used to produce lime mortars and plasters due to its plasticity and ability to harden through carbonation. Hydraulic lime contains lime silicates which allow it to set and harden even when submerged in water.