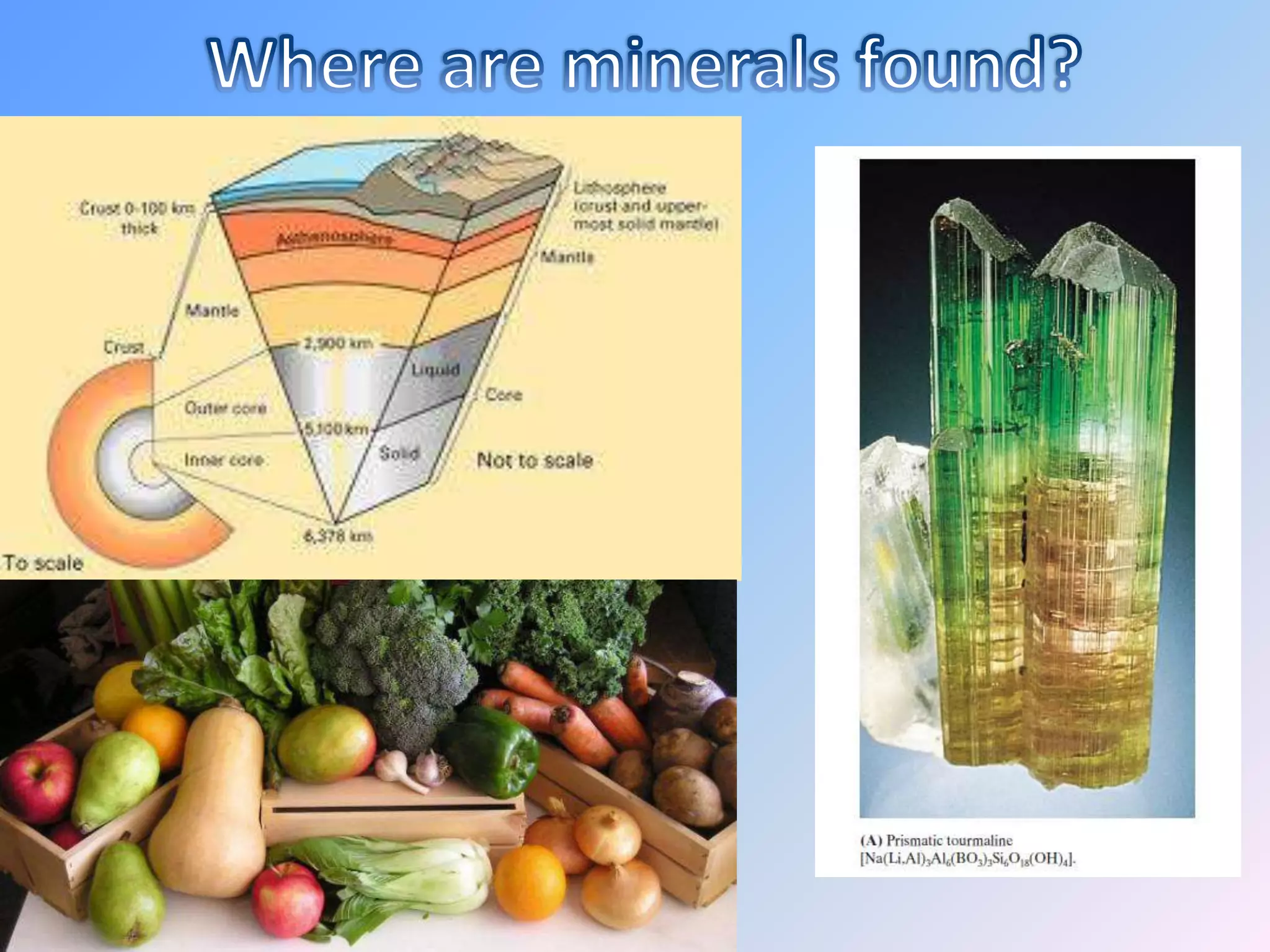
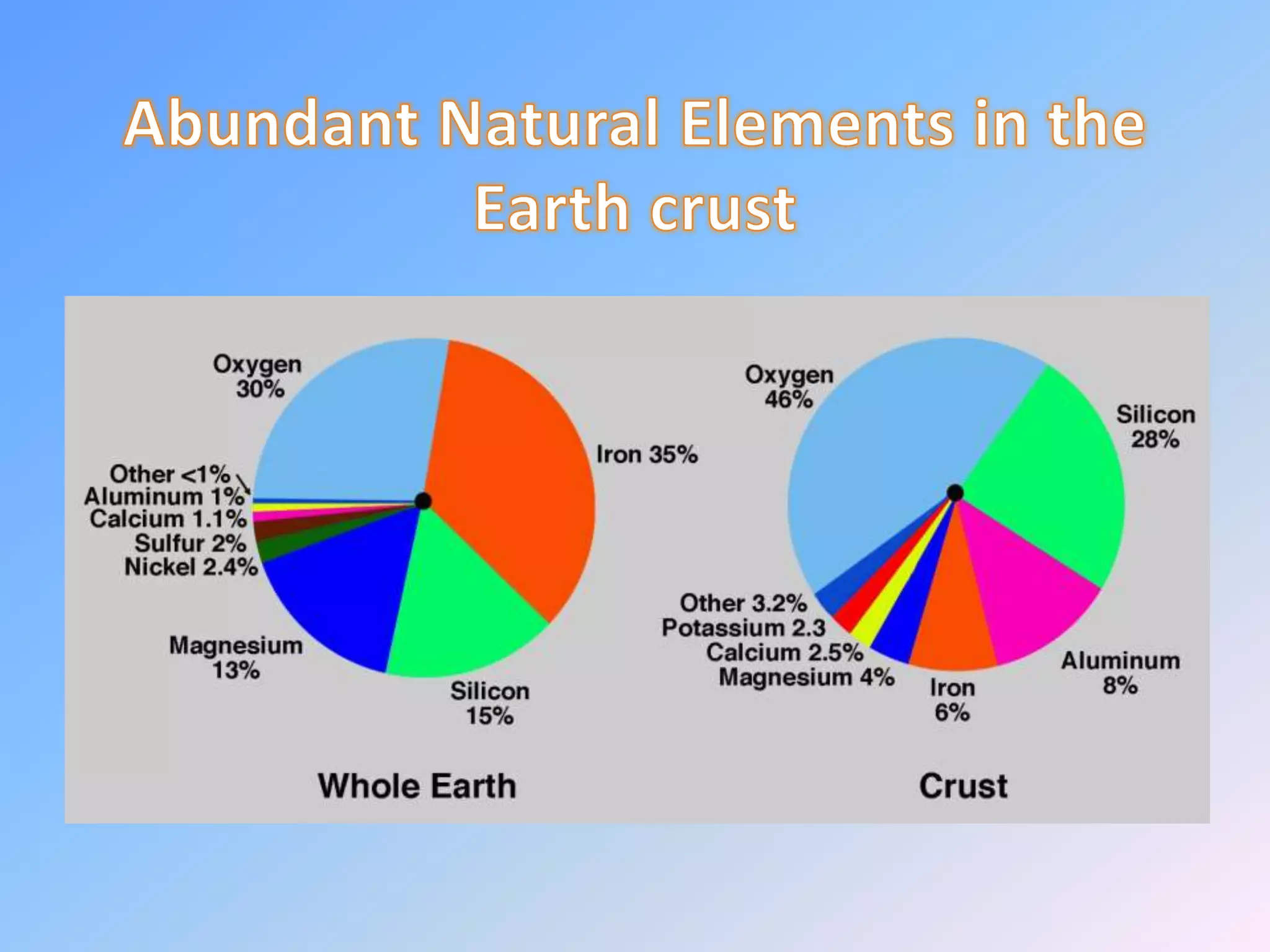
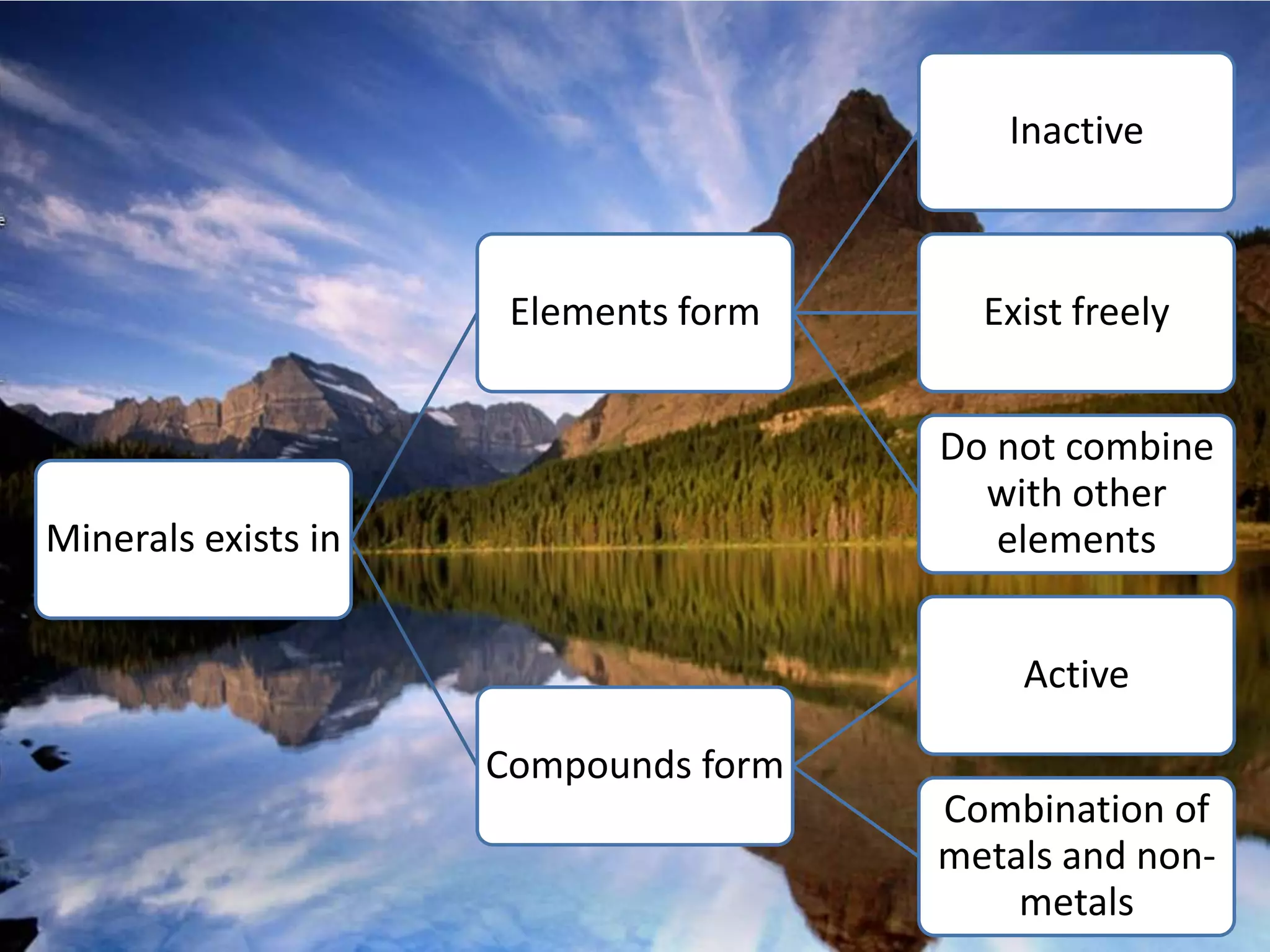
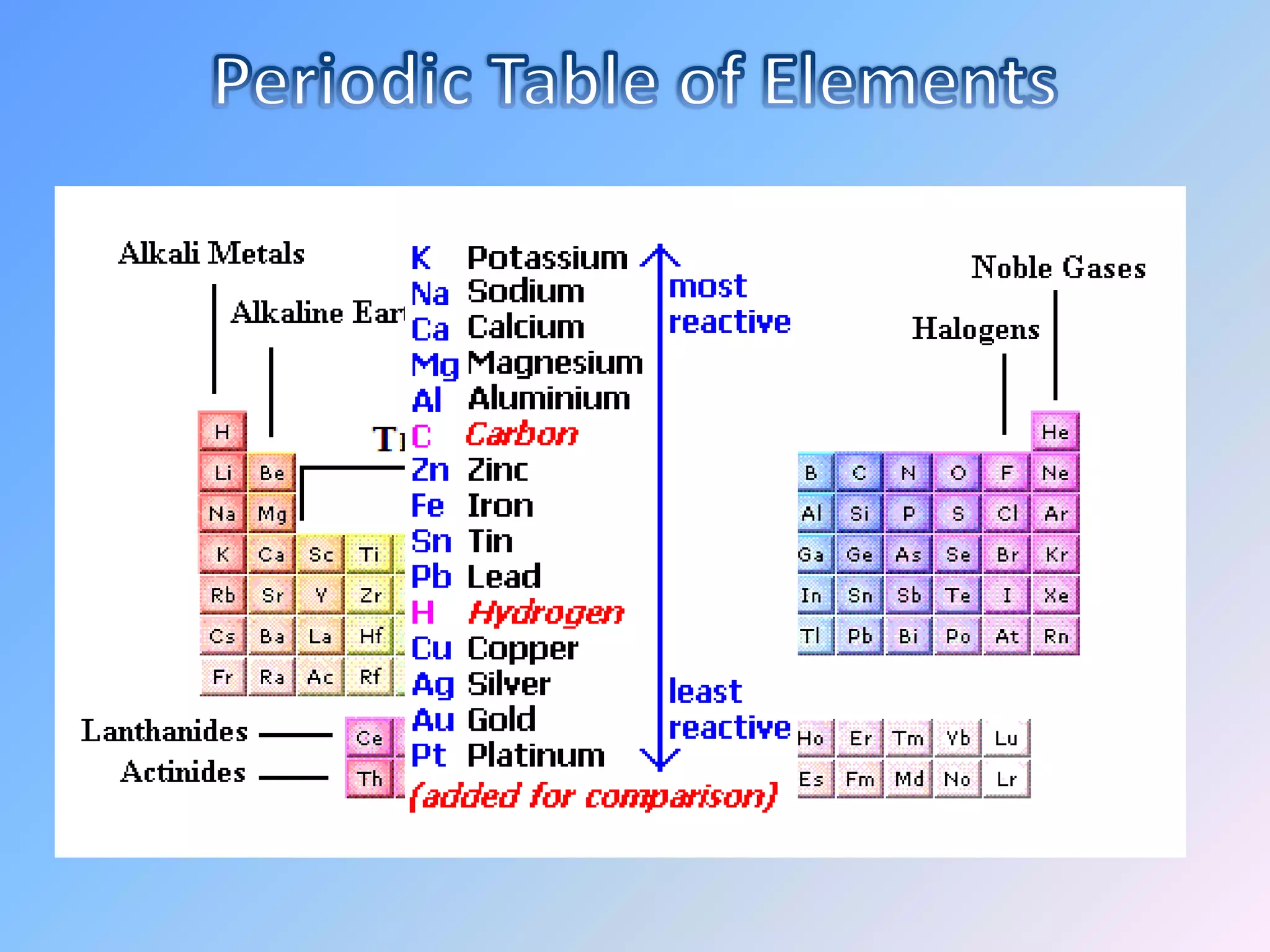
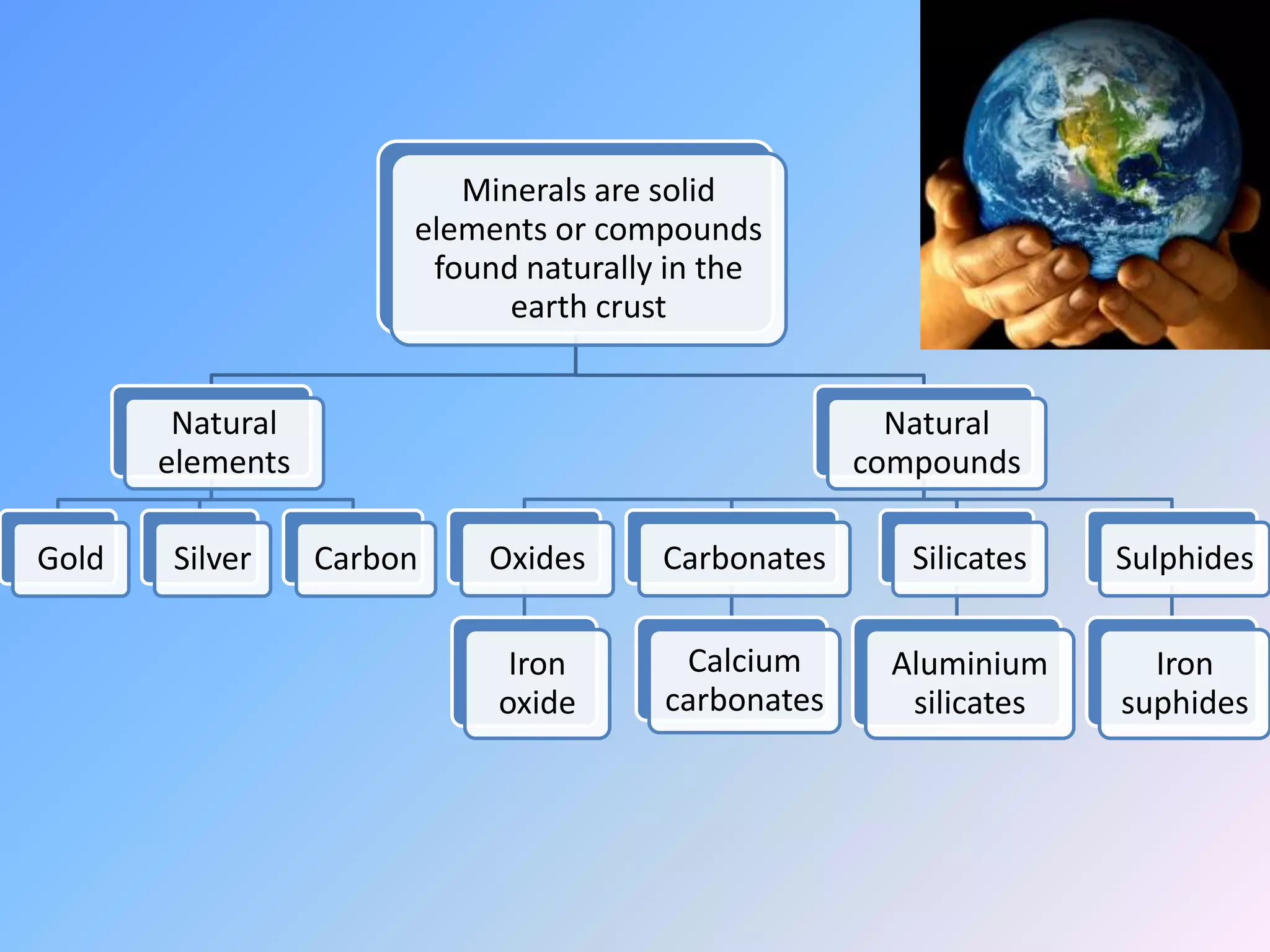
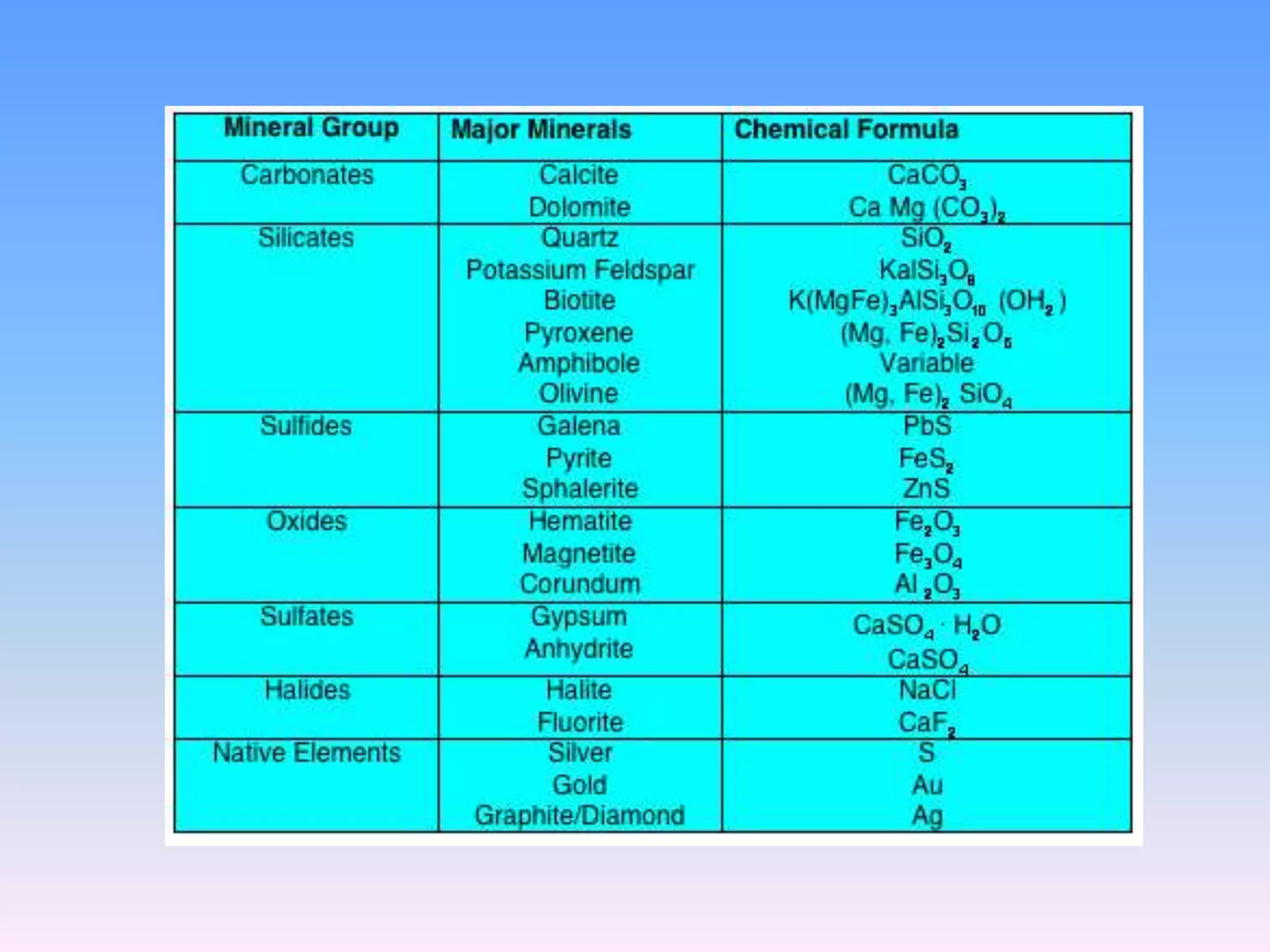
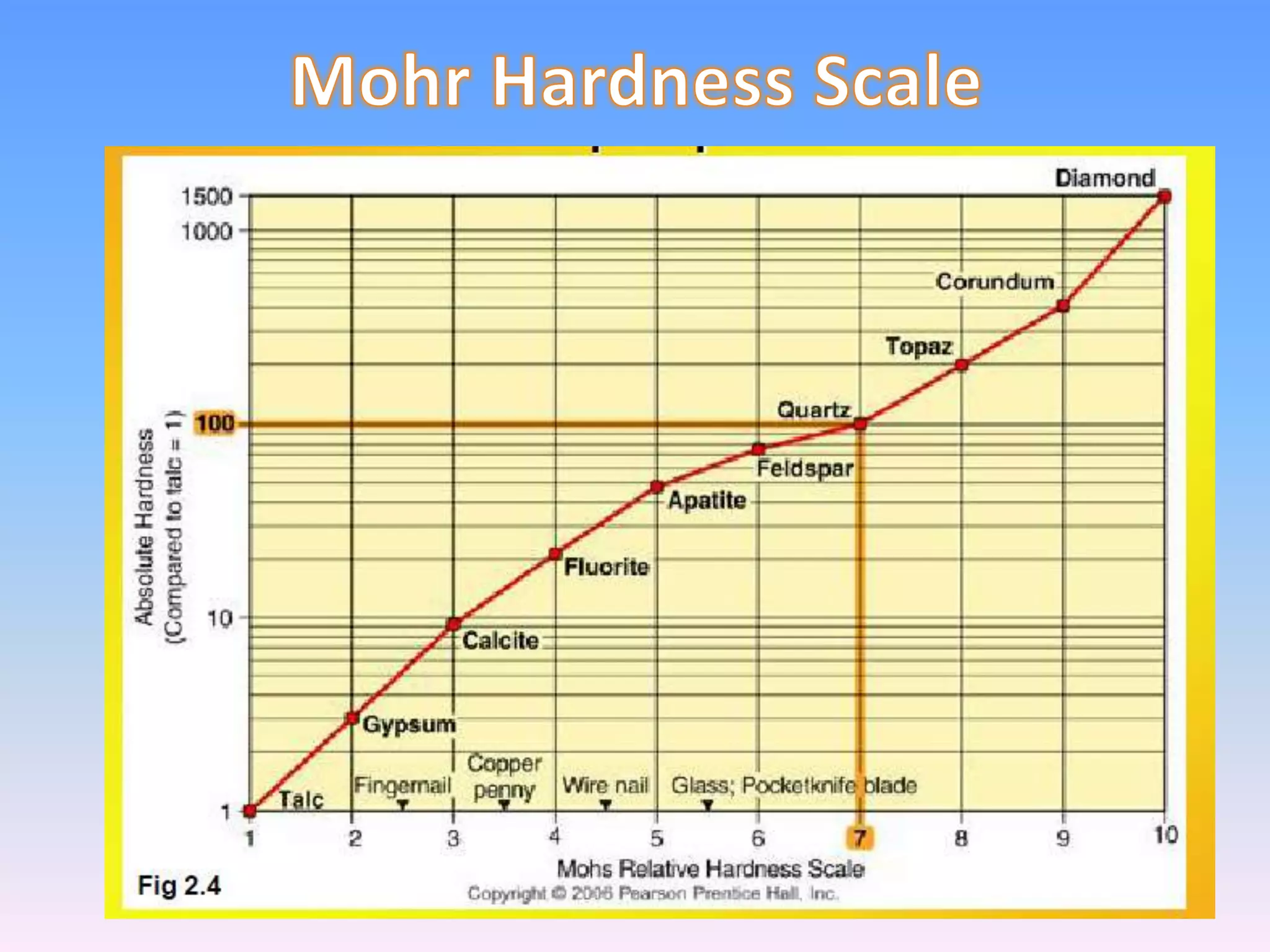
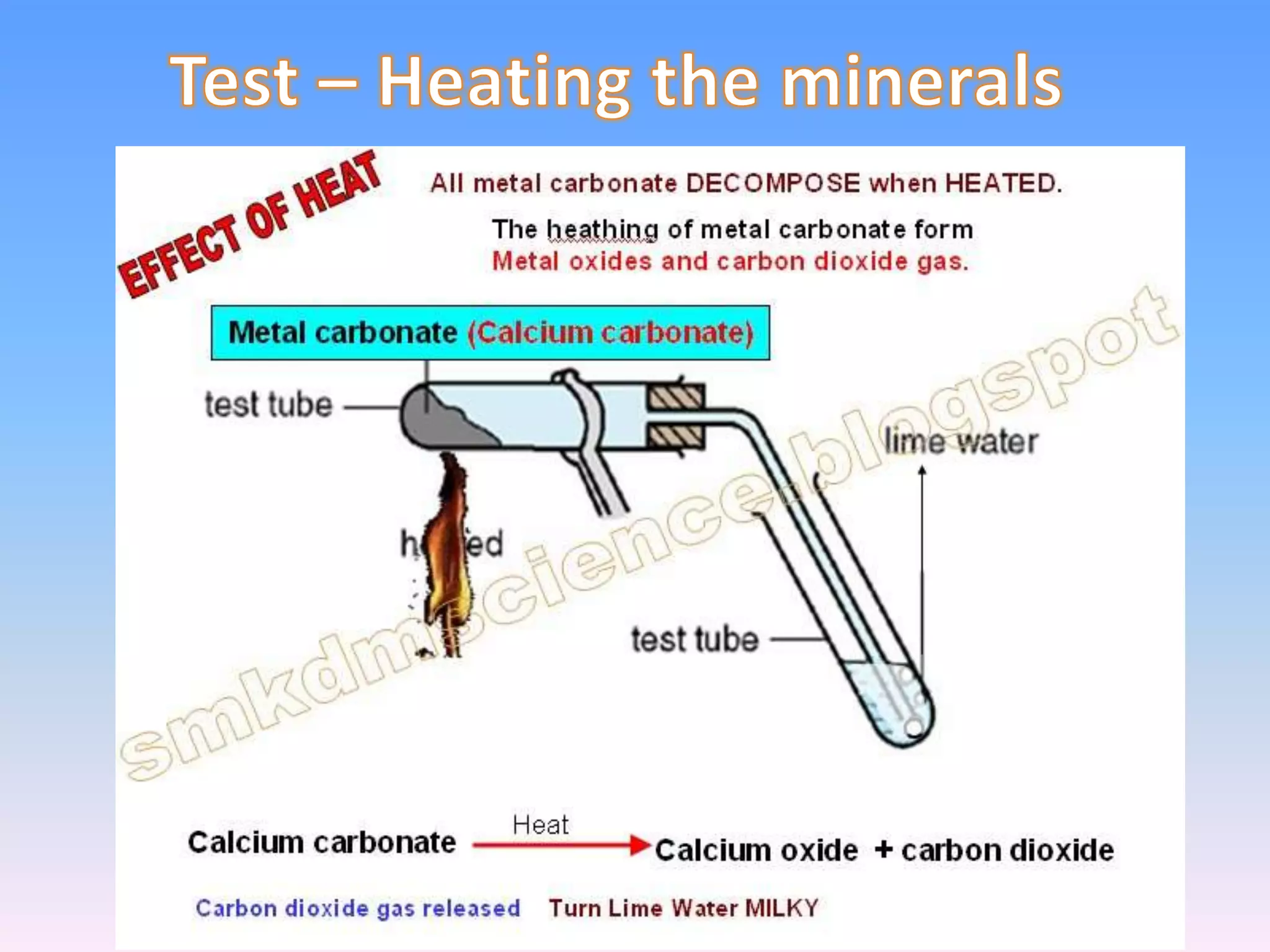
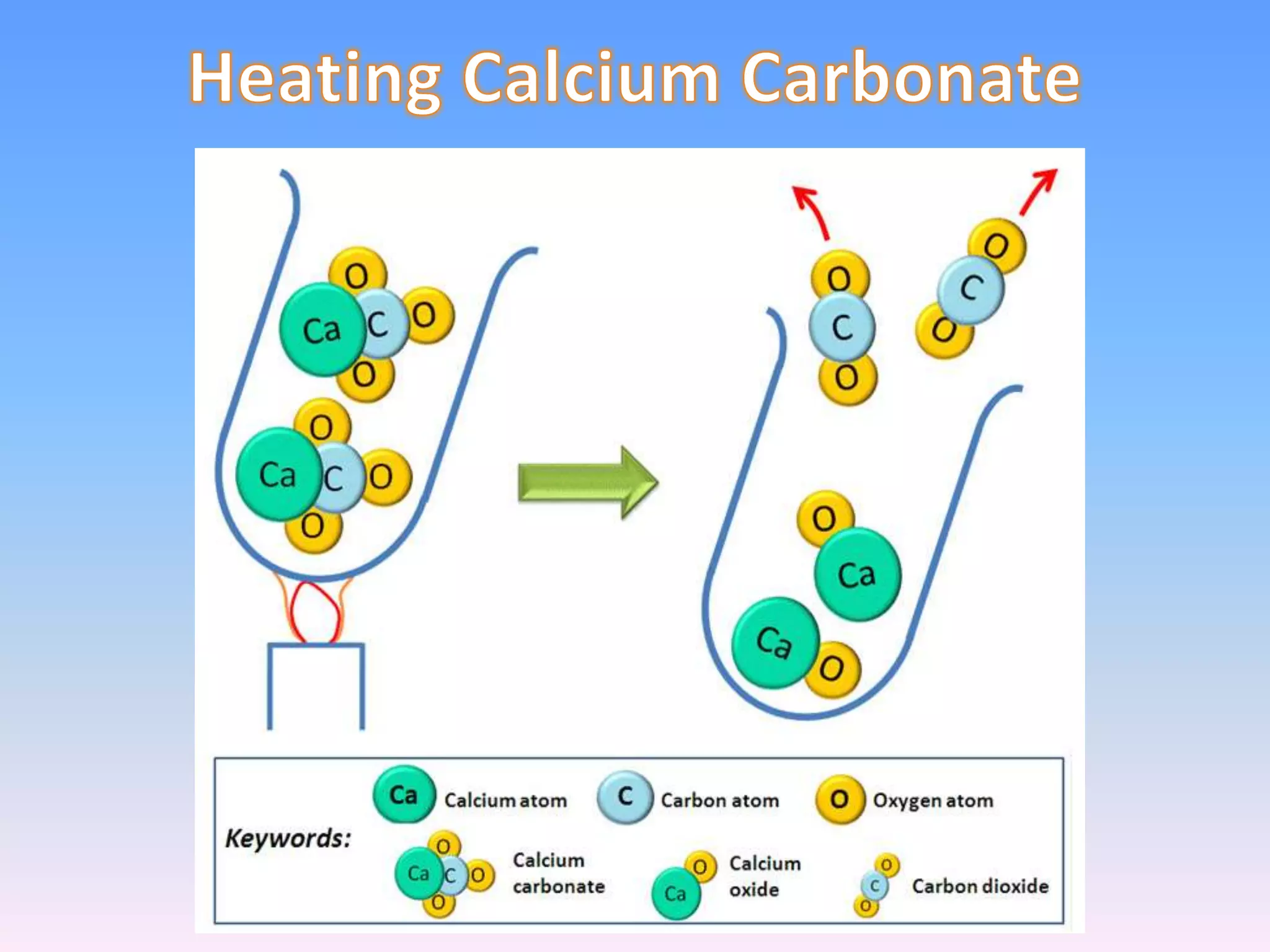
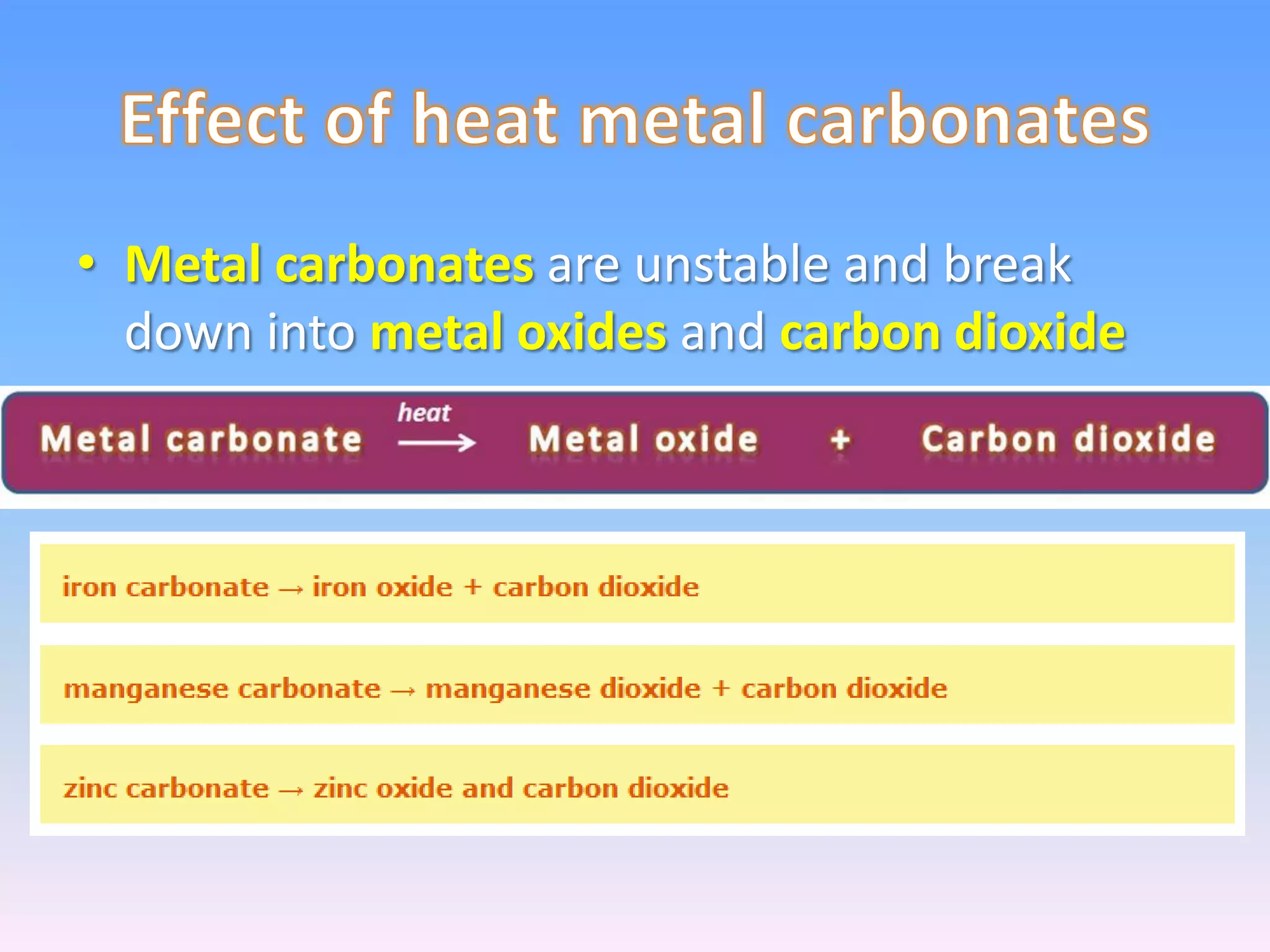
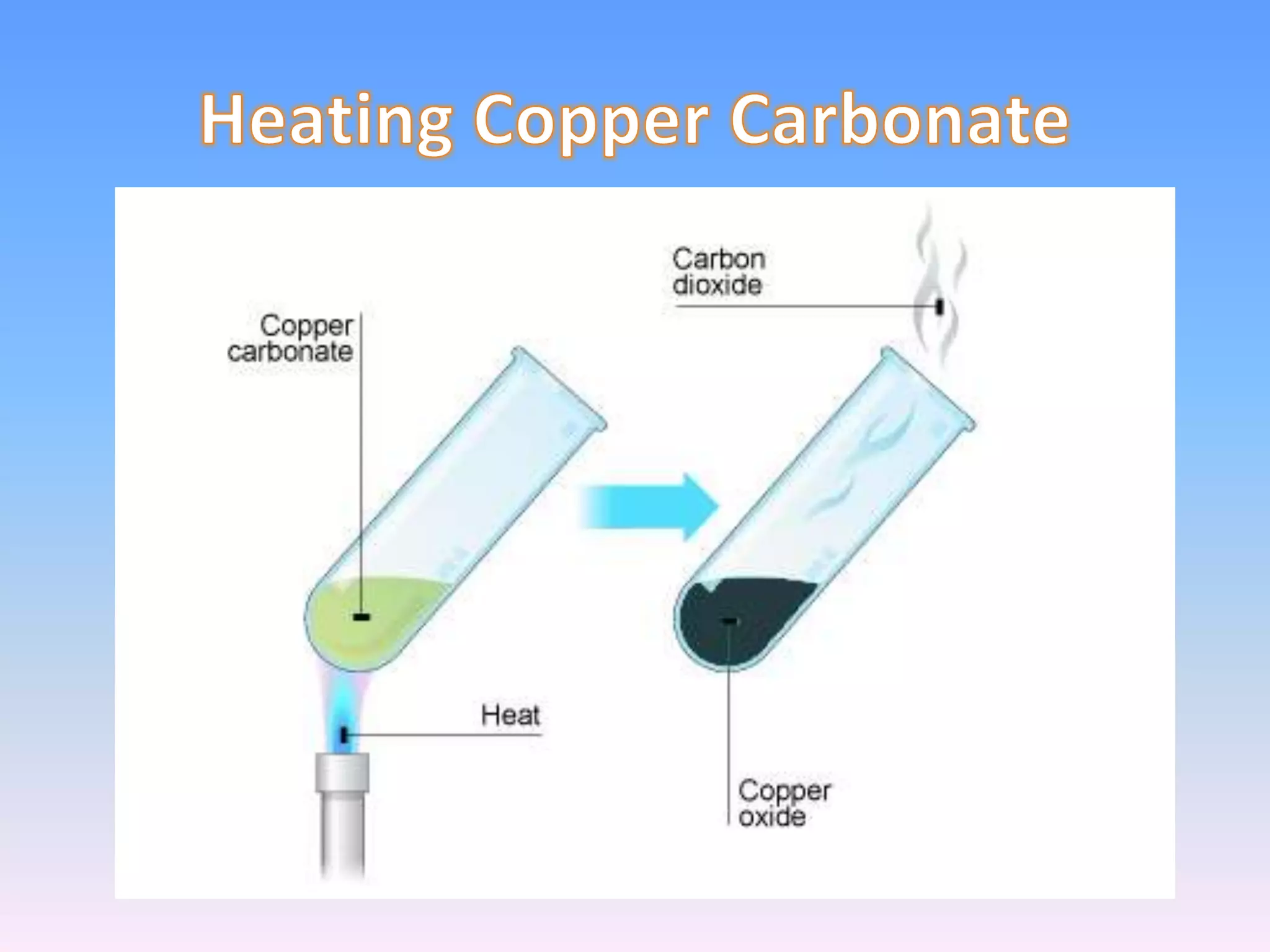
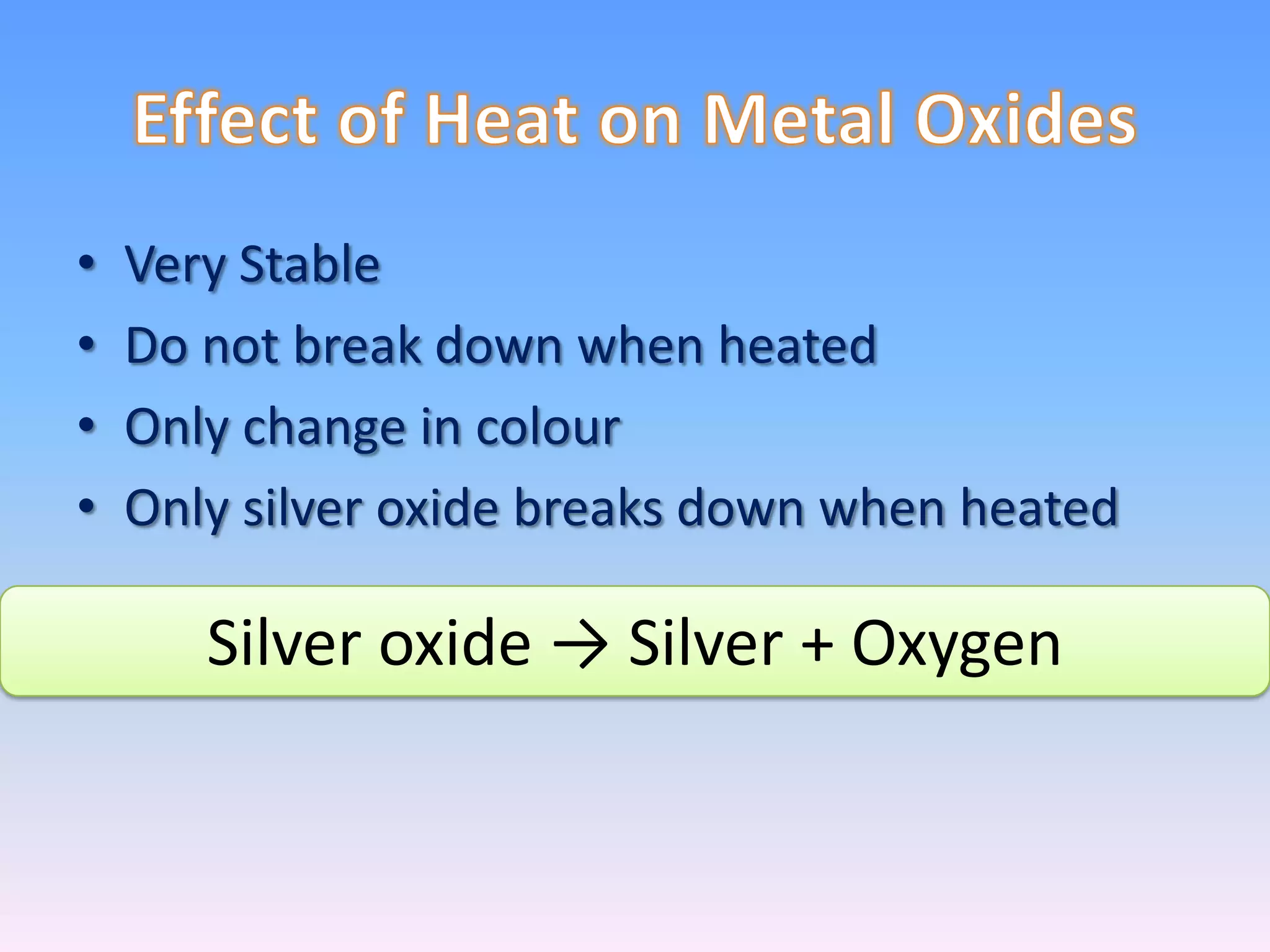
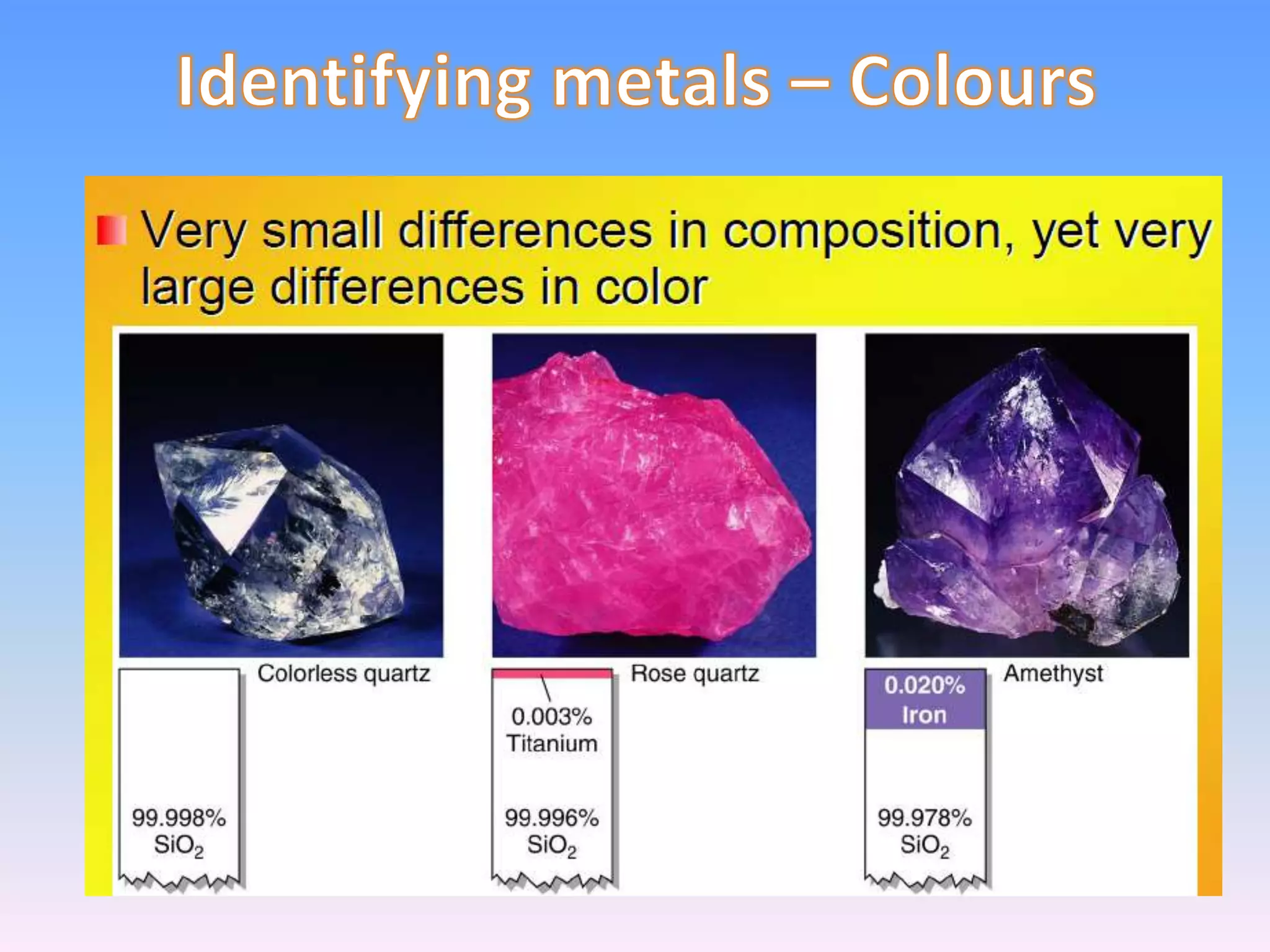
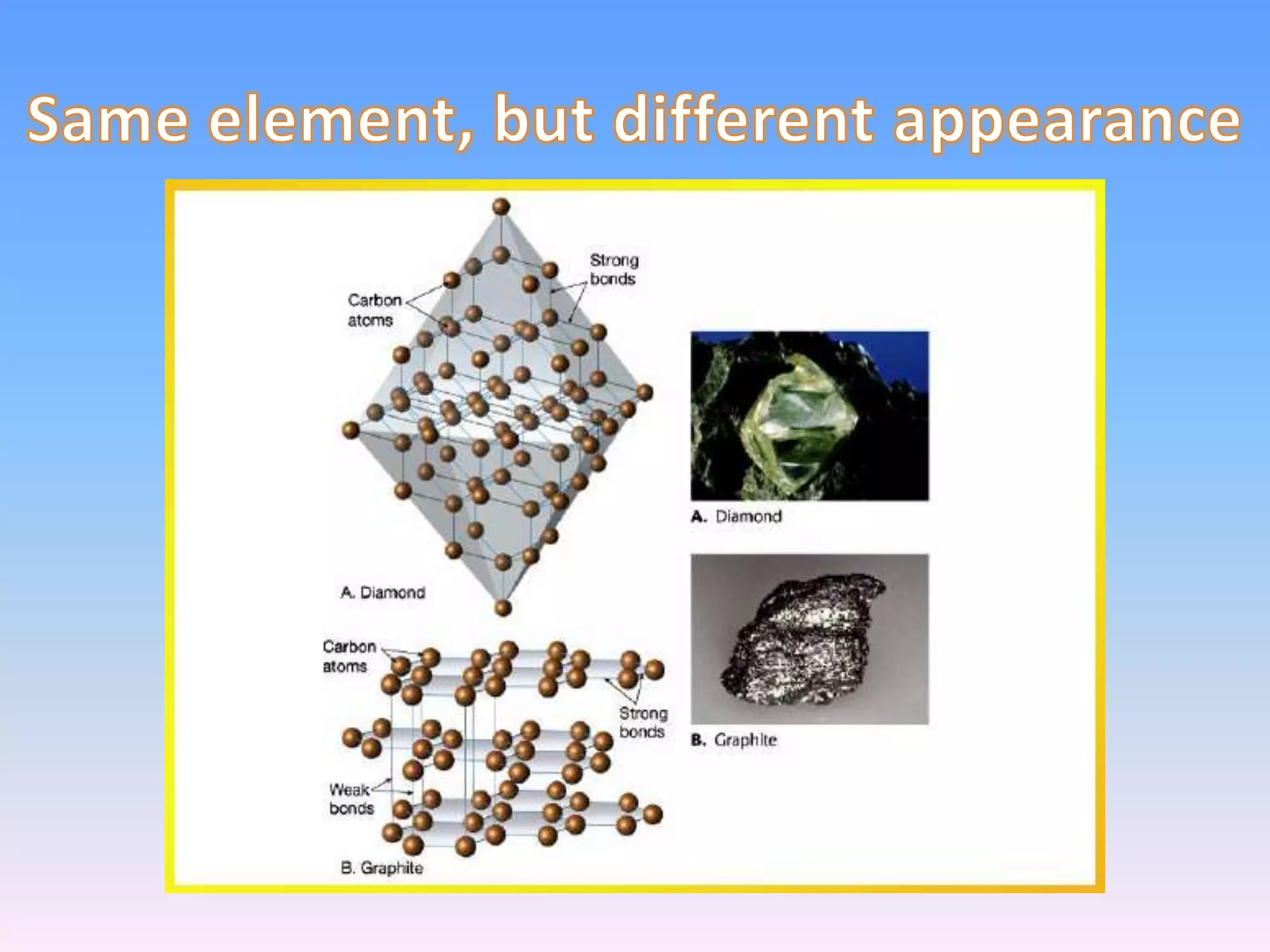
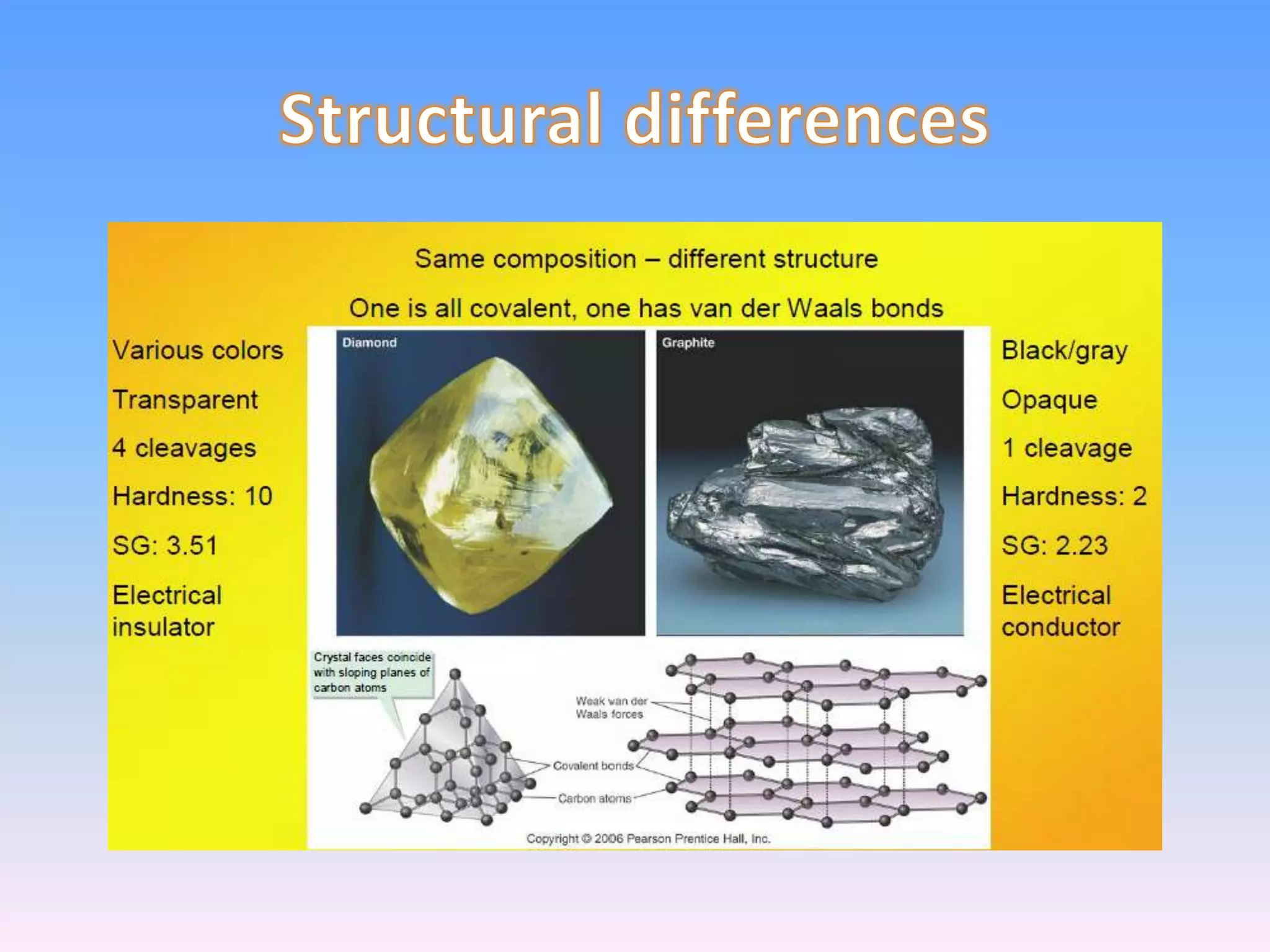
Minerals are naturally occurring inorganic solids with a definite chemical composition and structure. They can exist as elements like gold or silver or as compounds like oxides, carbonates, and silicates. Minerals form in two ways - elements exist freely in nature while compounds form through the combination of elements and minerals.