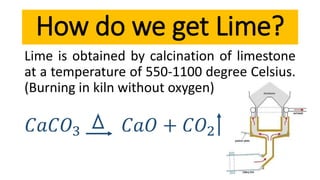
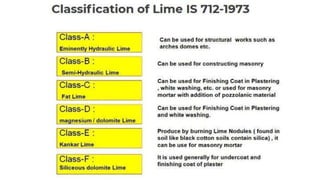
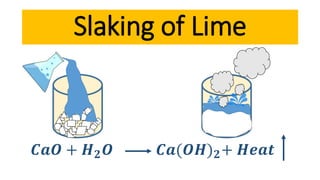
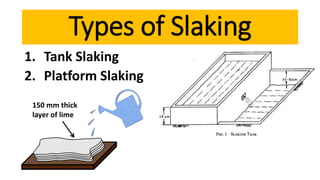
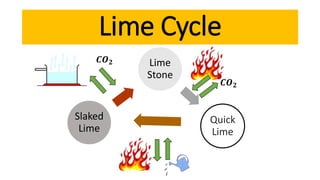
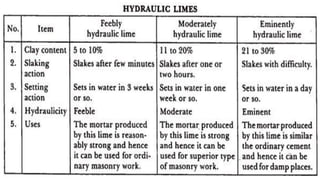
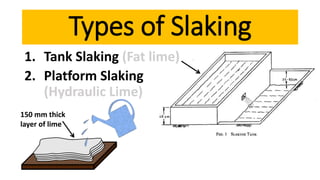
Lime is a construction material obtained by calcining limestone and has been used since ancient times for building structures such as palaces and temples. It is known for its binding properties, flexibility, and resistance to moisture, with different types including fat lime, hydraulic lime, and poor lime, each with unique characteristics and uses. Common applications of lime include mortar for masonry, plastering, and soil stabilization.