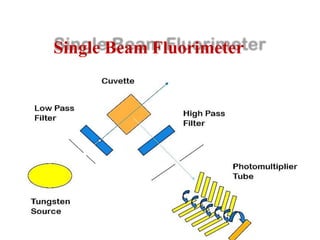
The document provides a comprehensive overview of fluorescence spectrophotometry, detailing the principles of luminescence, types of fluorescence (such as Stokes and anti-Stokes), and the factors affecting fluorescence intensity. It discusses the excited state processes in molecules, including collisional deactivation and intersystem crossing, and emphasizes how chemical structure and environmental conditions impact fluorescence. Additionally, it covers instrumentation used in fluorescence measurement and various applications across different fields.




















![Factors affecting Fluorescence
Extreme sensitiveness of the method requires very dilute
solution.
Adsorption of the fluorescent substances on the container
wall create serious problems.
Hence strong solutions must be diluted.
Light:
Monochromatic light is essential for the excitation of
fluorescence because the intensity will vary with wavelength.
Oxygen:
The presence of oxygen may interfere in 2 ways. 1] by direct
oxidation of the fluorescent substances to non fluorescent. 2]
by quenching of fluorescence.](https://image.slidesharecdn.com/1unit2-240807111337-dfe616ae/85/1-UNIT-2-pdf-26-320.jpg)