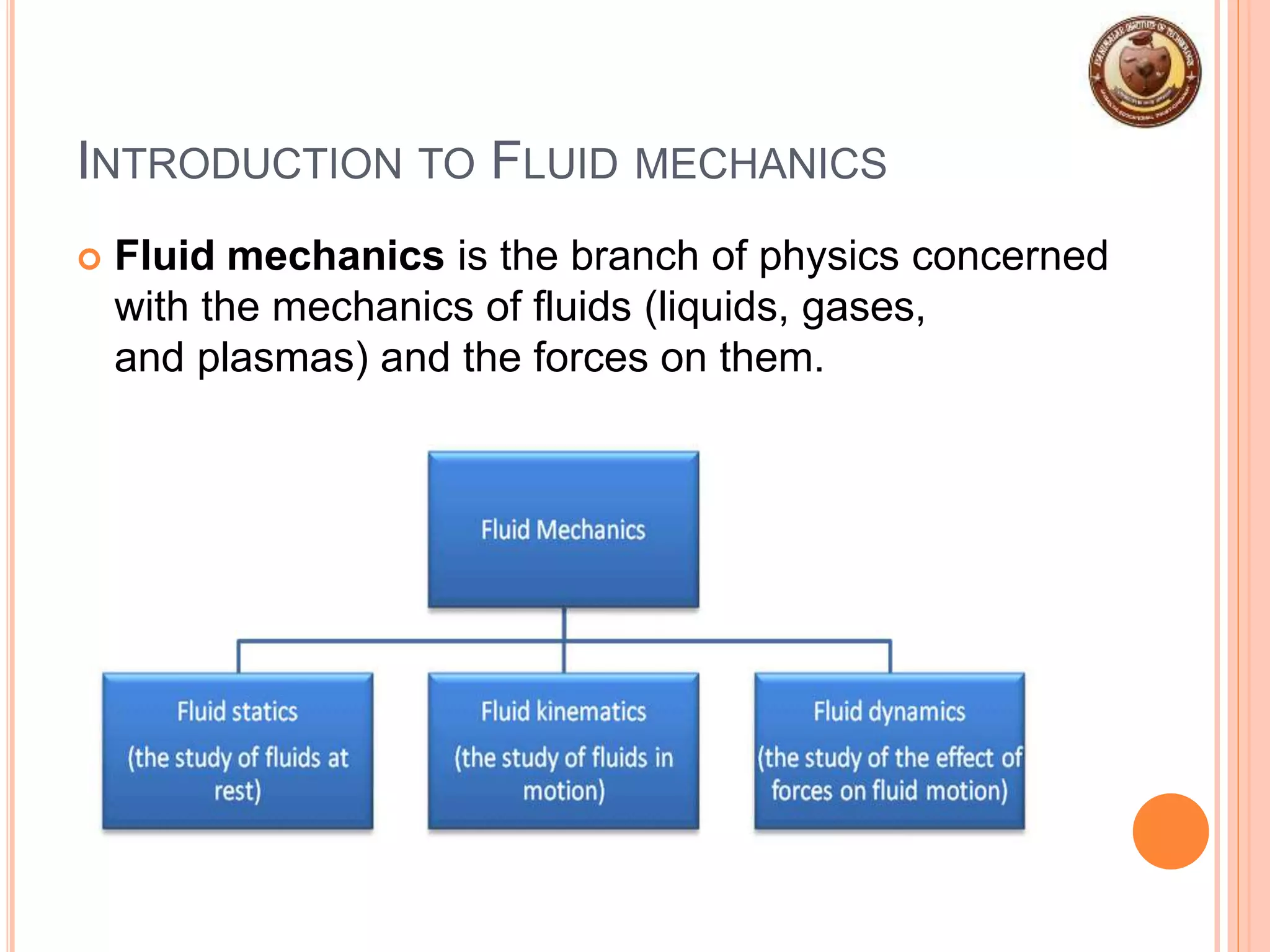
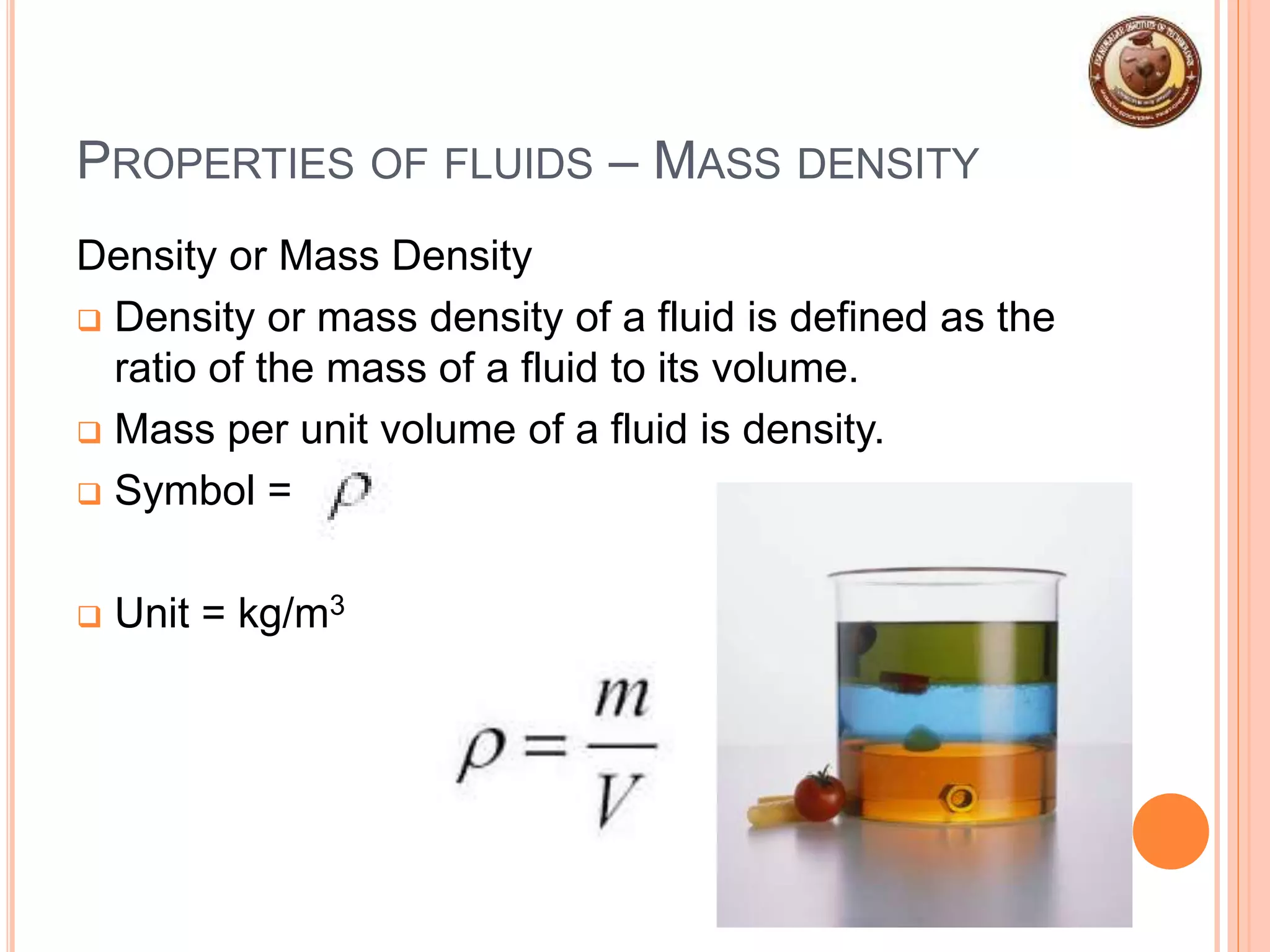
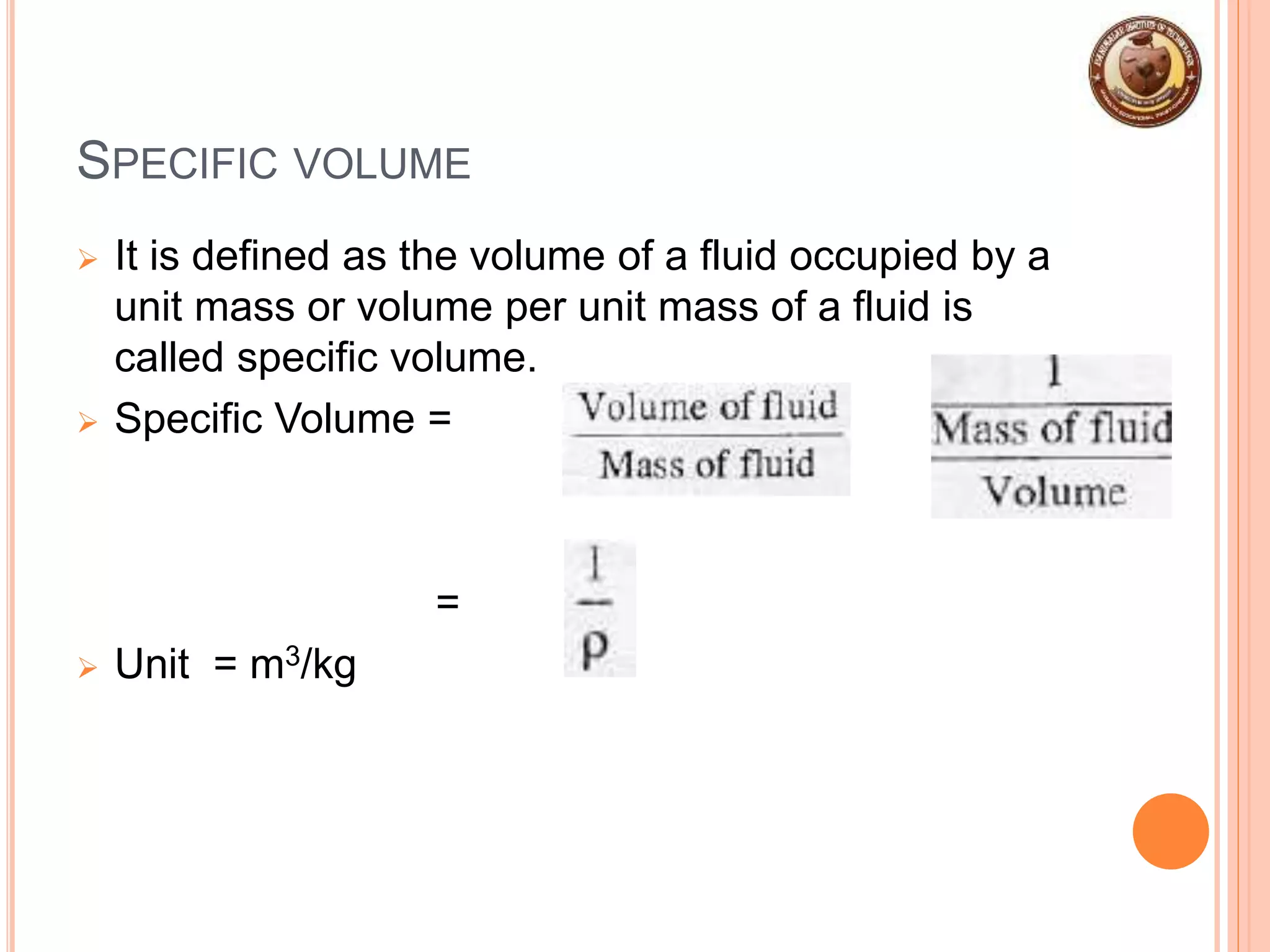
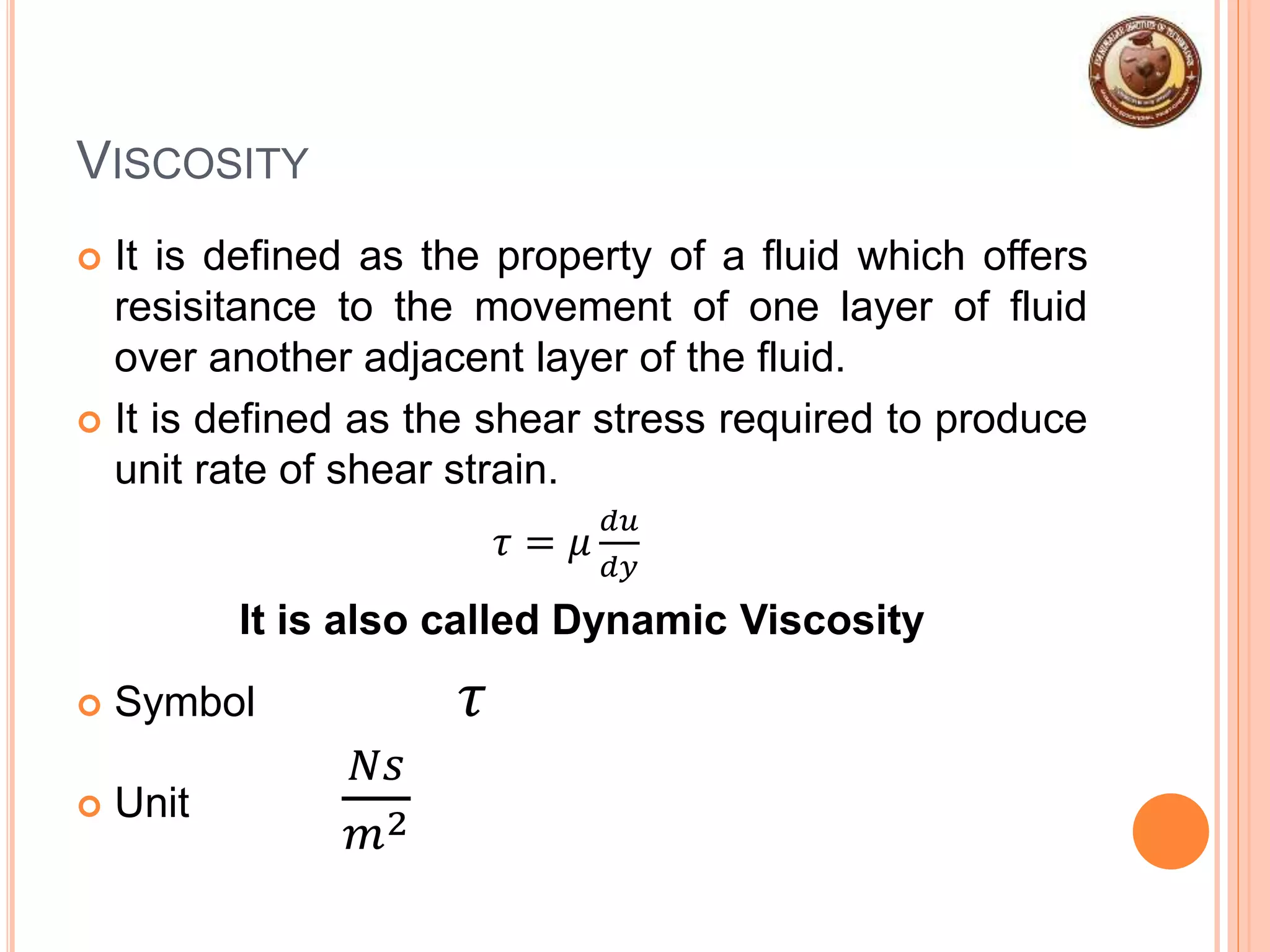
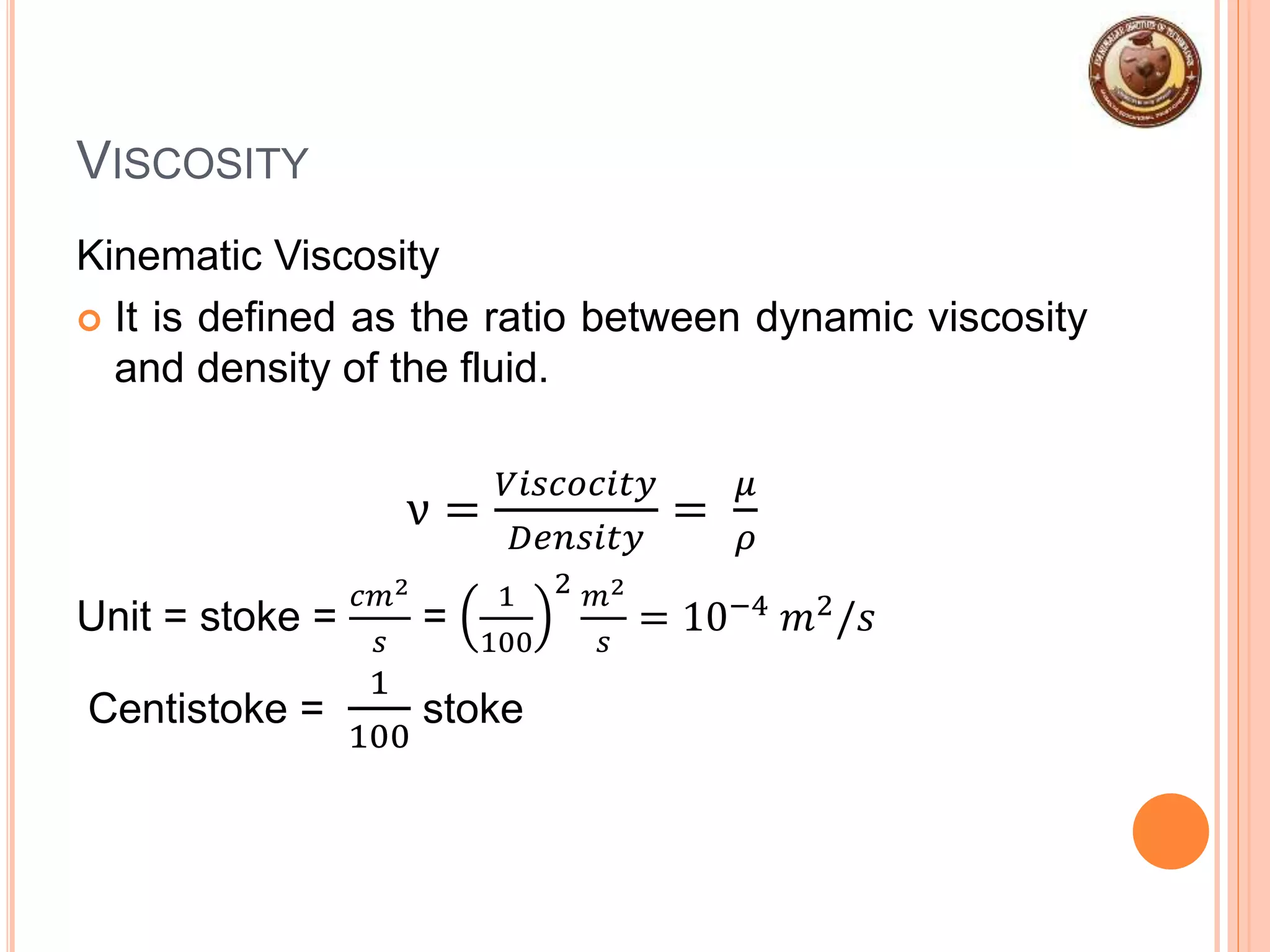
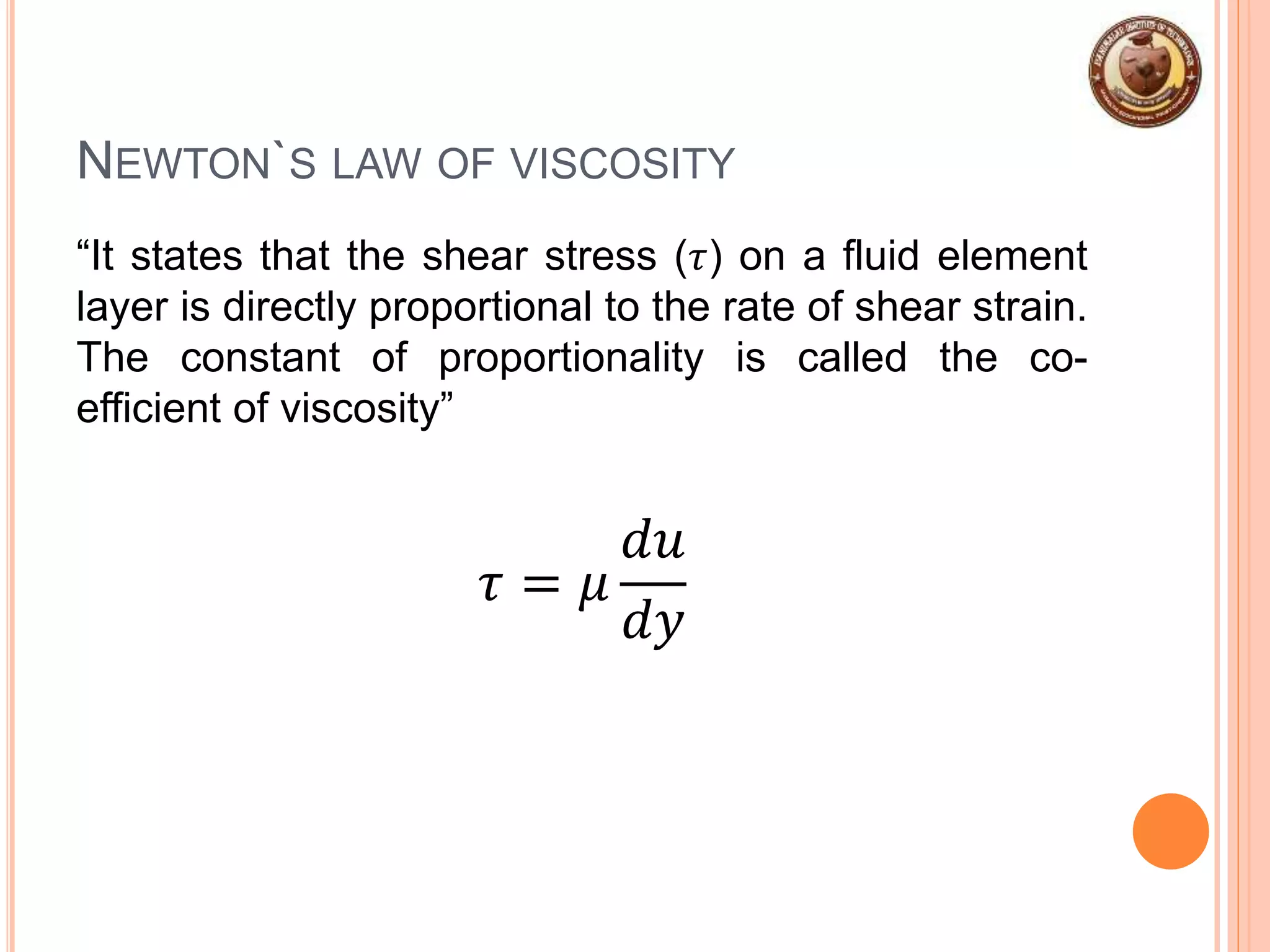
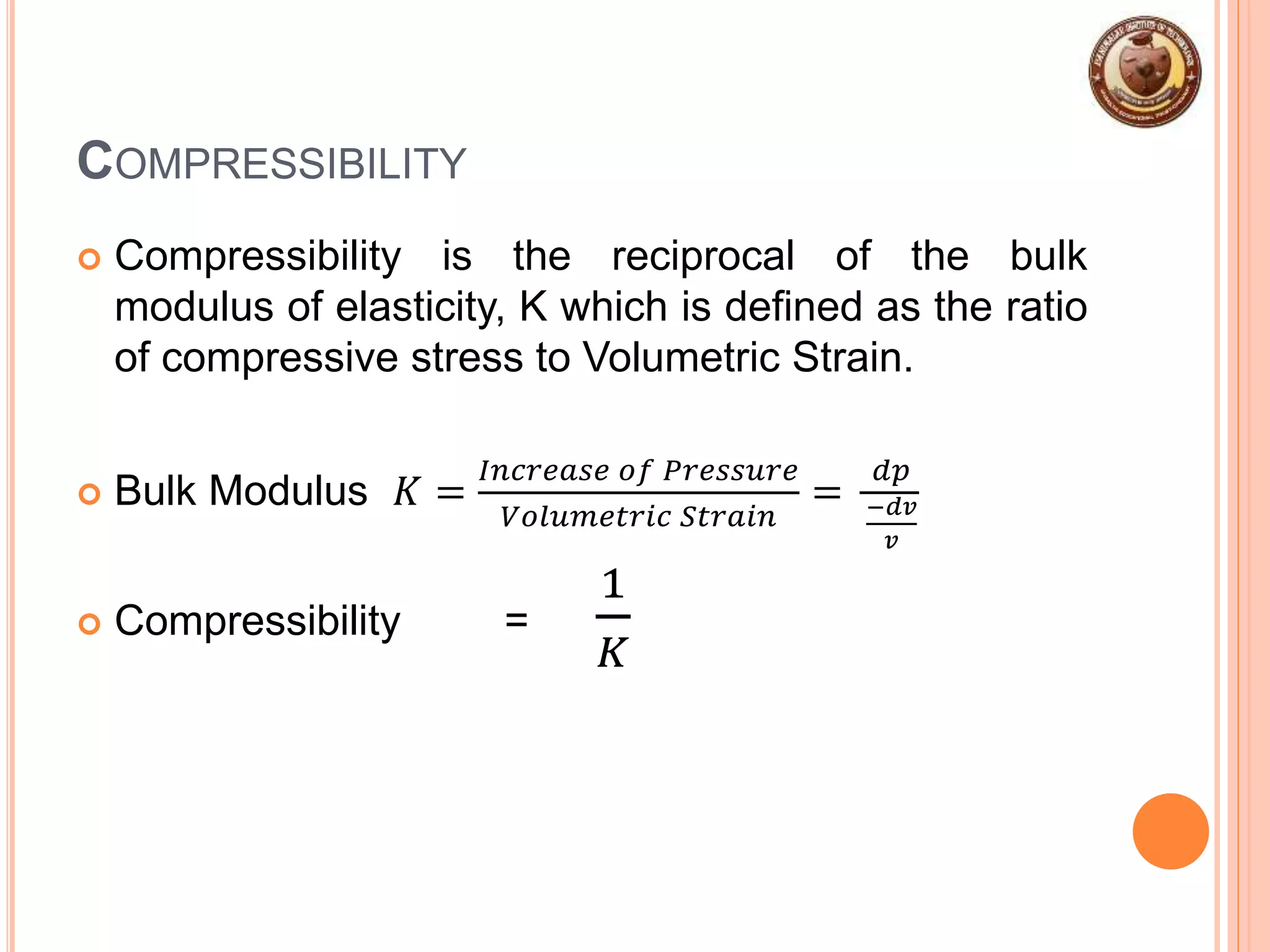
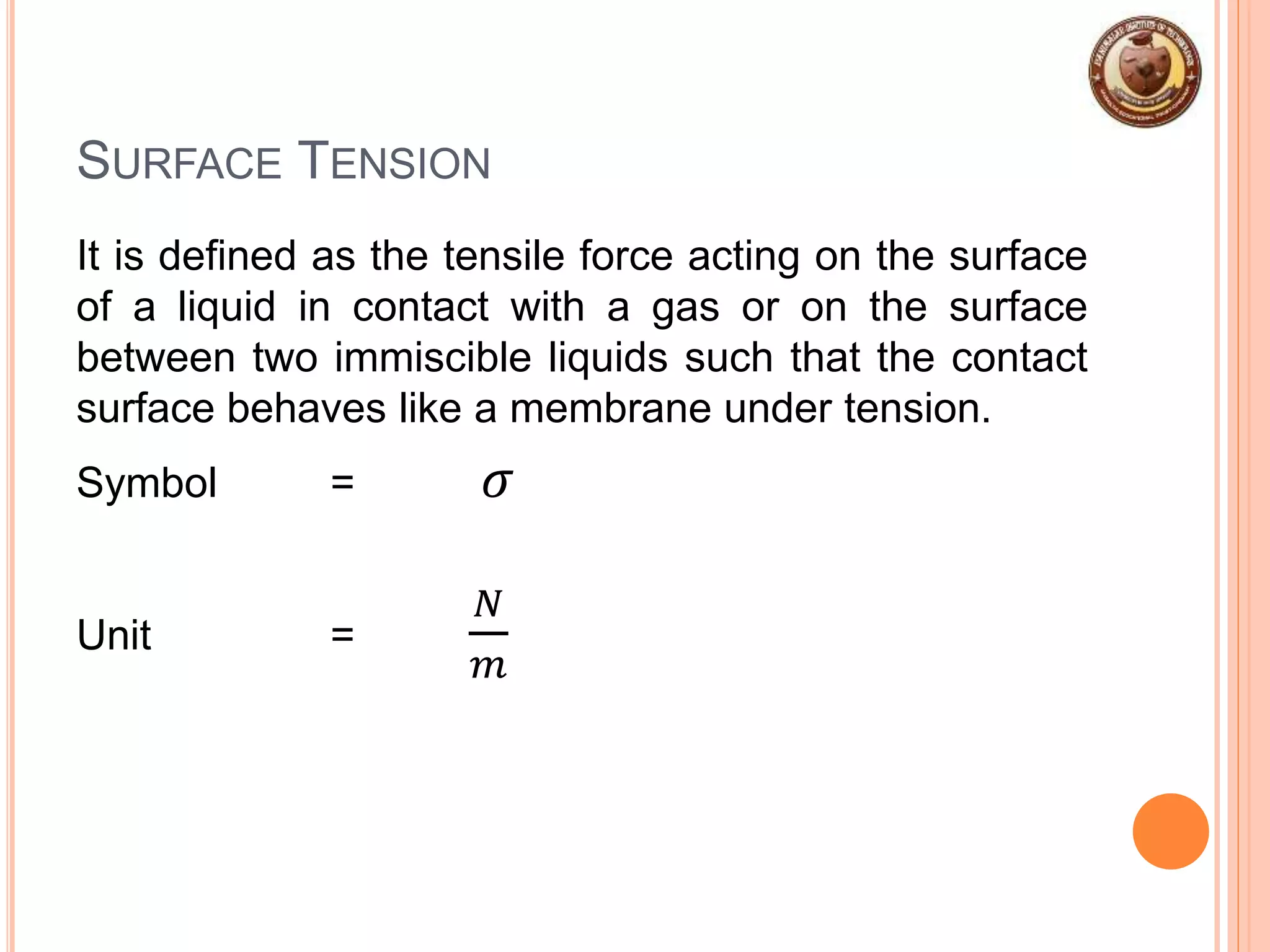
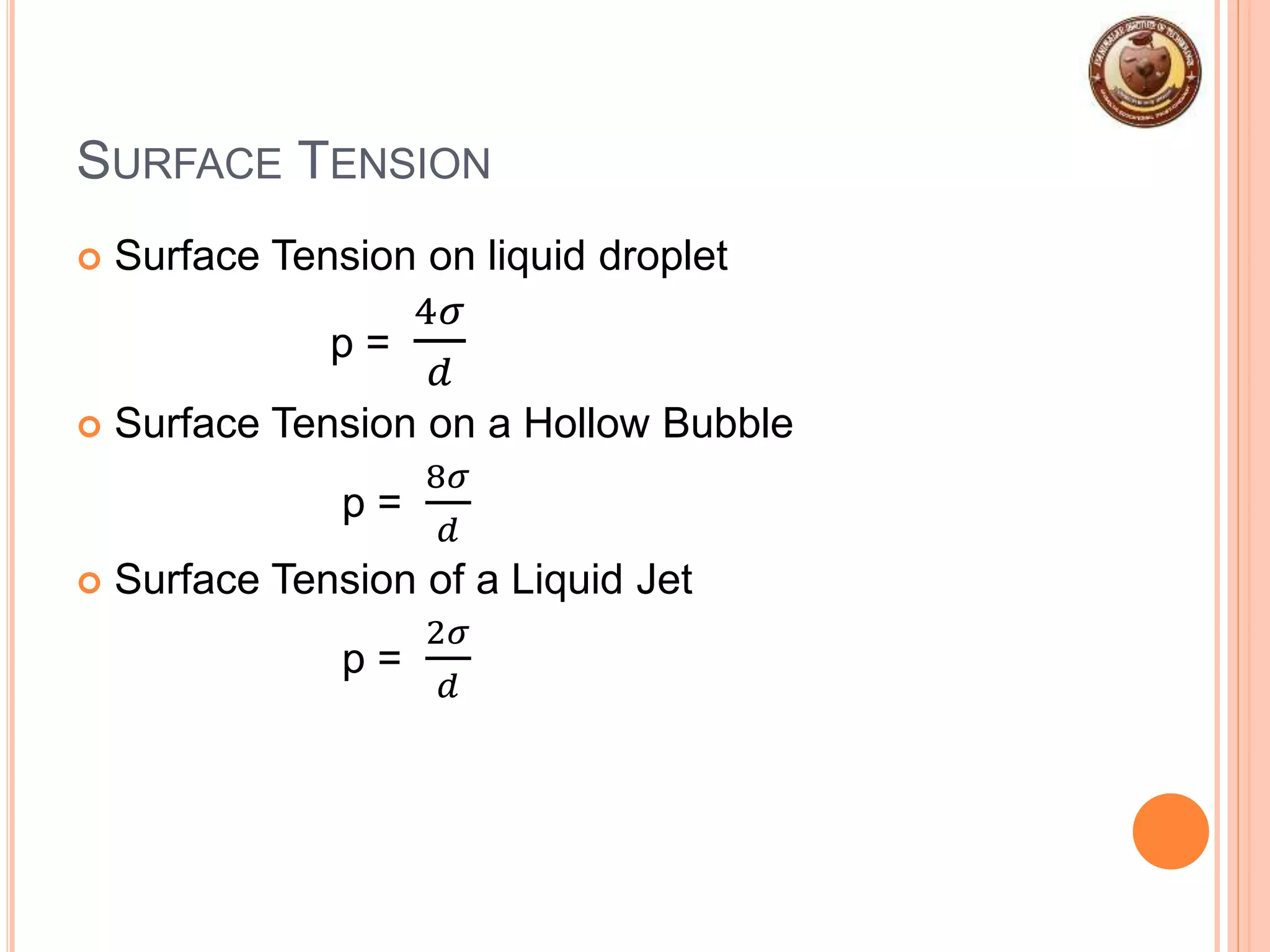
This document provides an overview of fluid mechanics and fluid properties. It introduces units and dimensions used to describe fluids, then defines key fluid properties like density, viscosity, compressibility, and surface tension. Various fluid types are described, including ideal, real, Newtonian and non-Newtonian fluids. Fundamental concepts in fluid mechanics like Newton's law of viscosity and vapor pressure are also summarized. The document is intended as an introduction to fluid mechanics and the characterization of fluids.