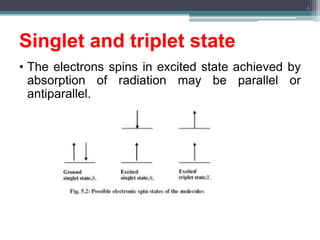
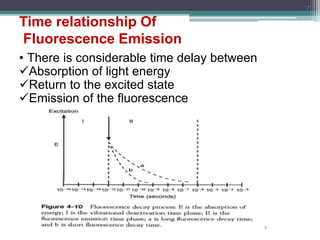
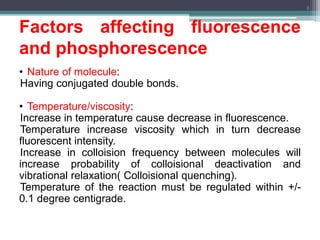
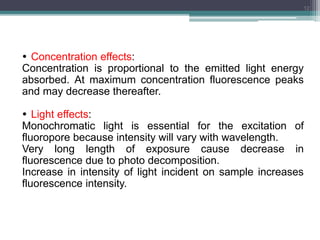
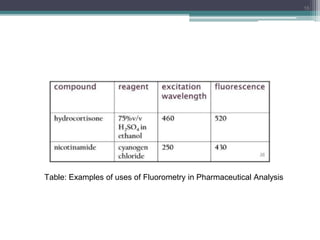
The document presents an introduction to fluorimetry, covering fundamental concepts such as luminescence, fluorescence, and phosphorescence, along with the molecular theories behind them. It discusses factors affecting fluorescence, including molecular structure, temperature, pH, and solvent characteristics, as well as practical applications in pharmaceutical and organic analysis. Key points also include the sensitivity of the method and the impact of different substituents on fluorescence intensity.