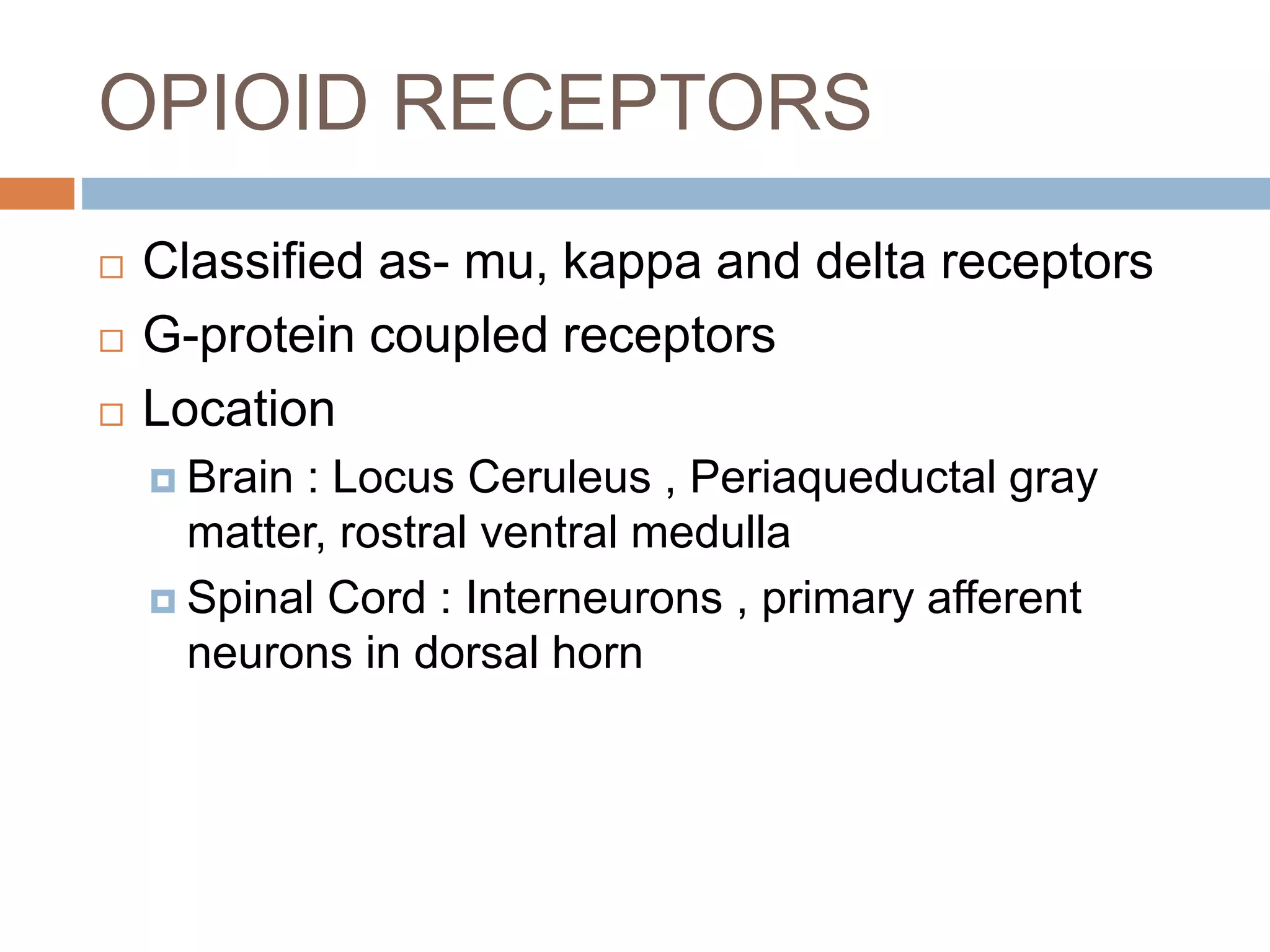
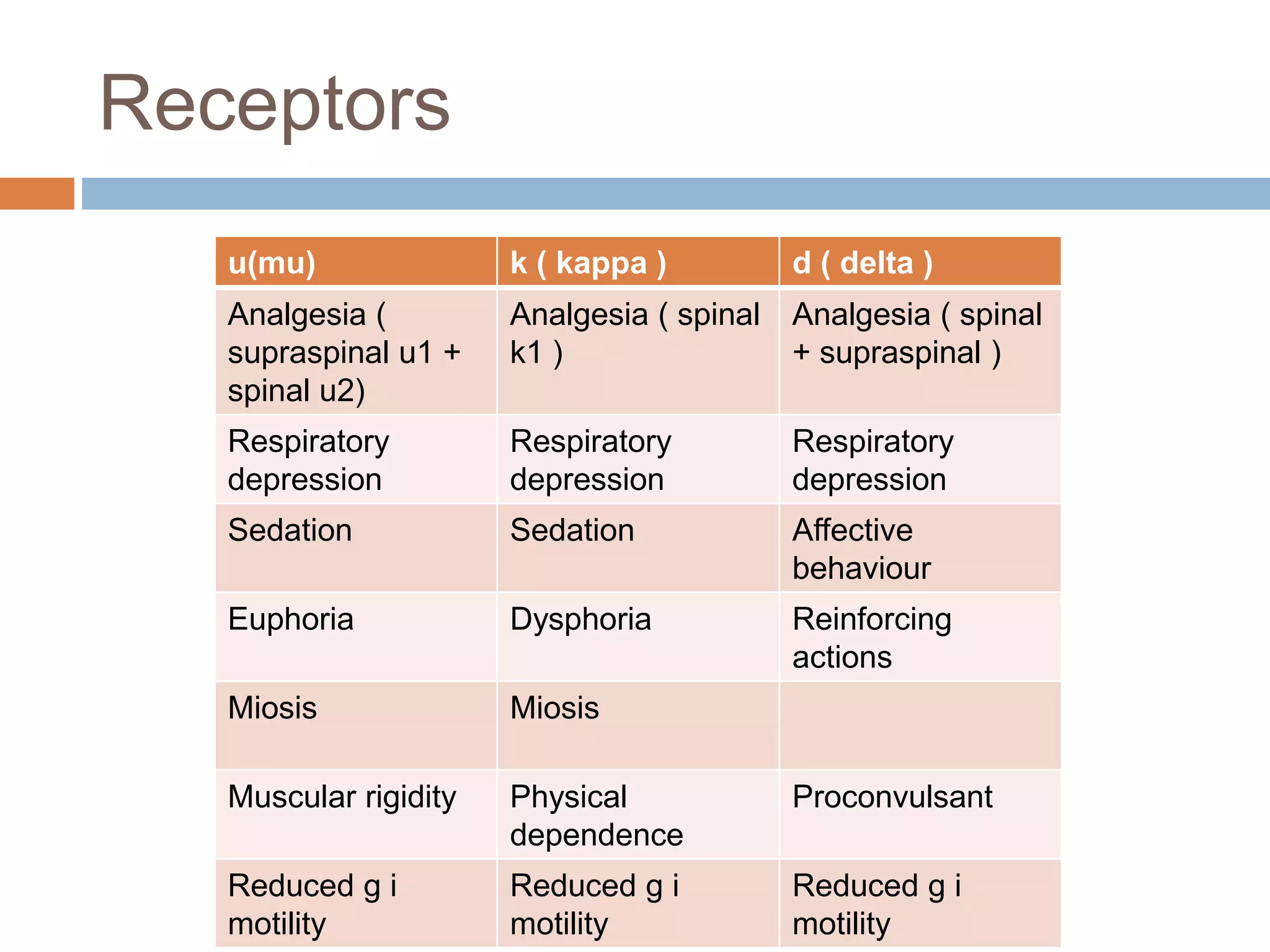
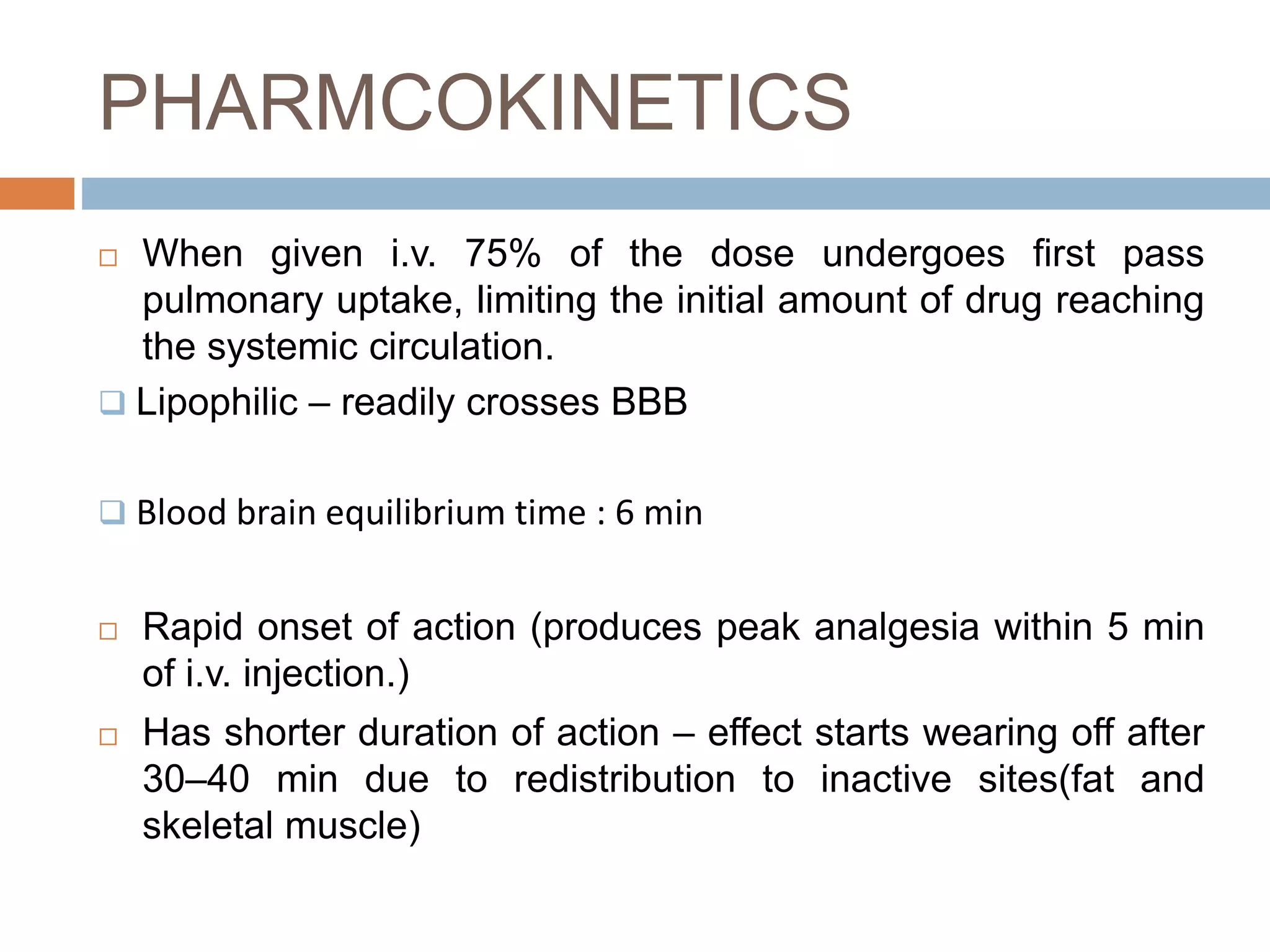
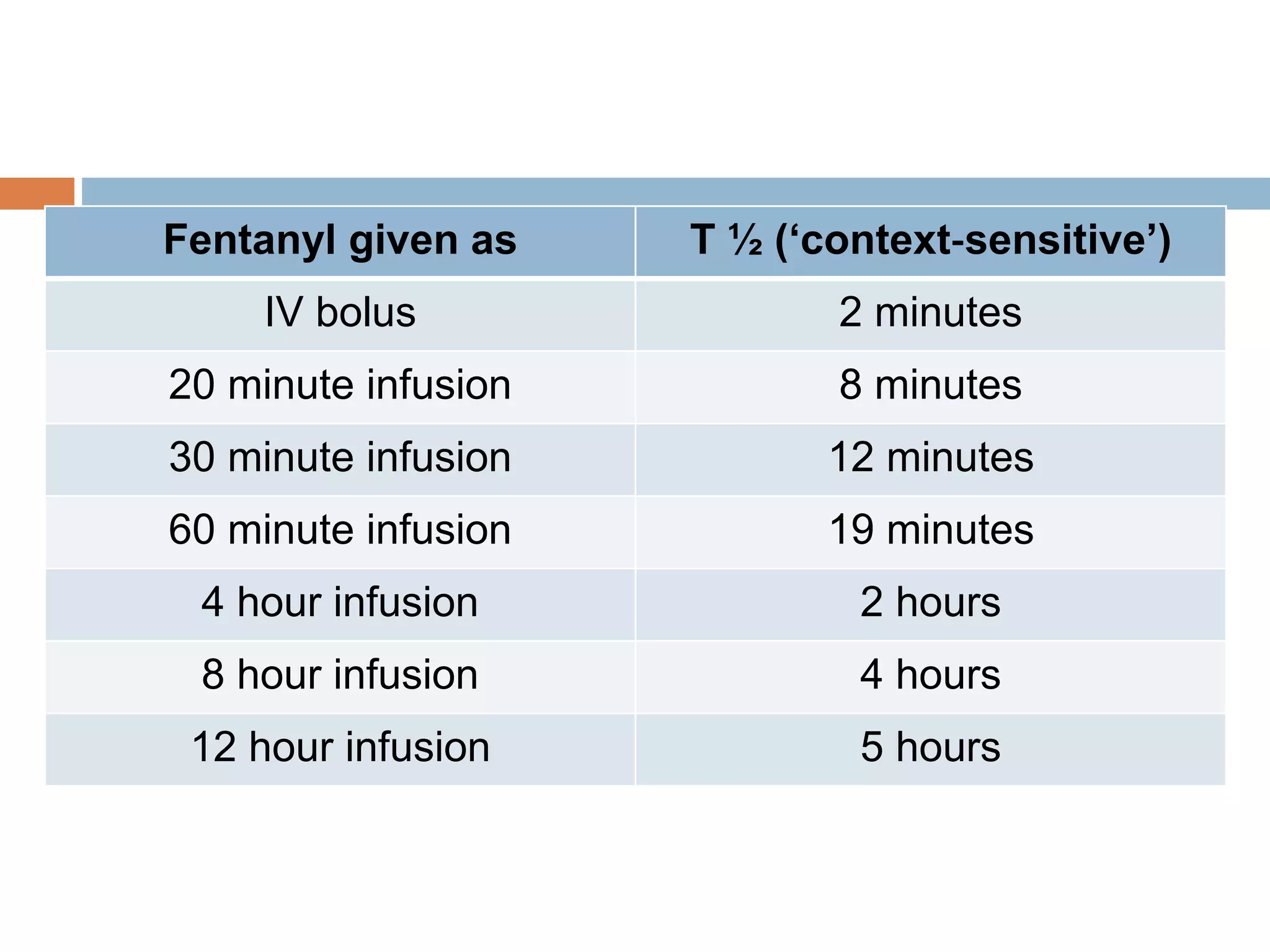
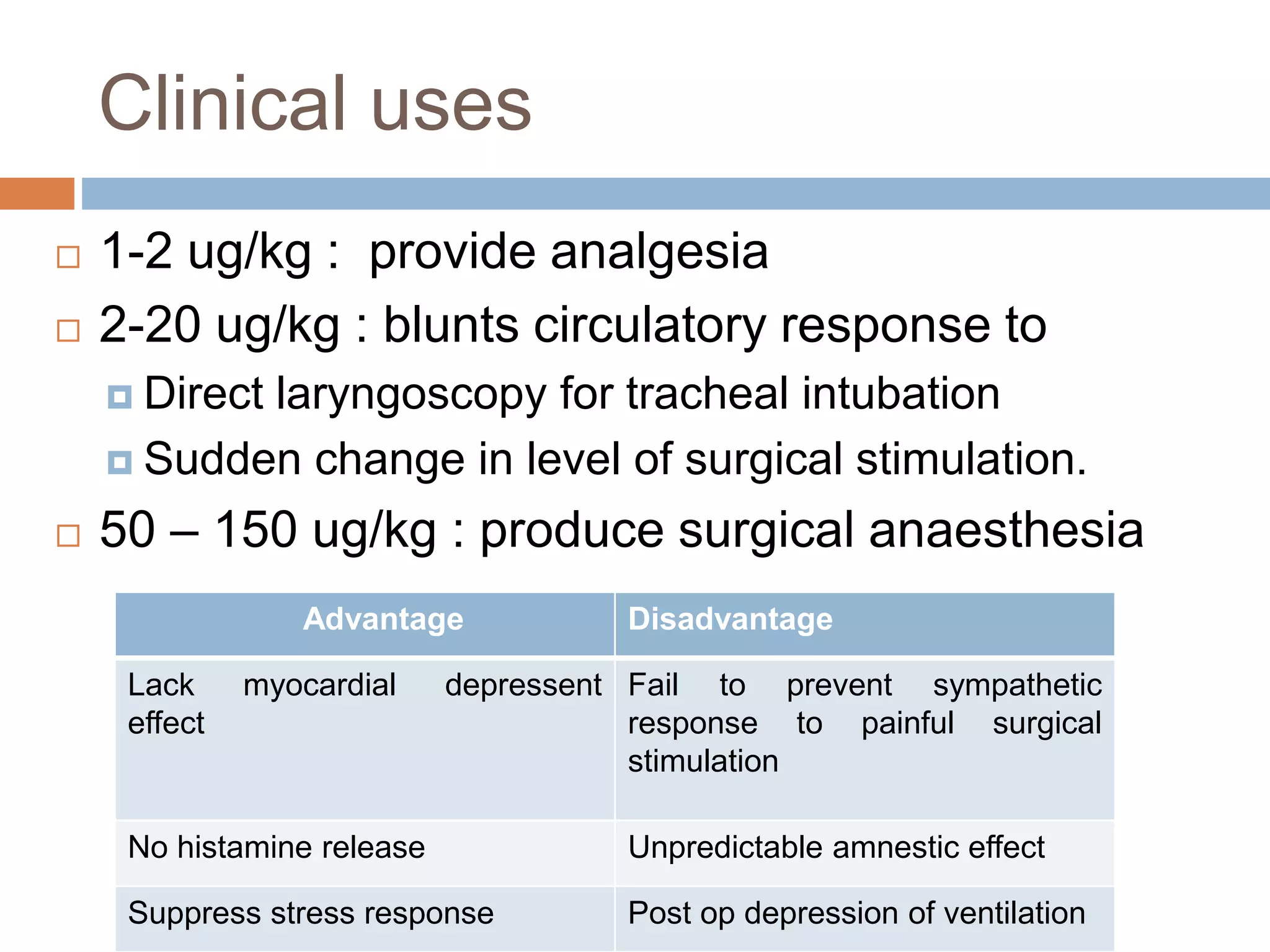
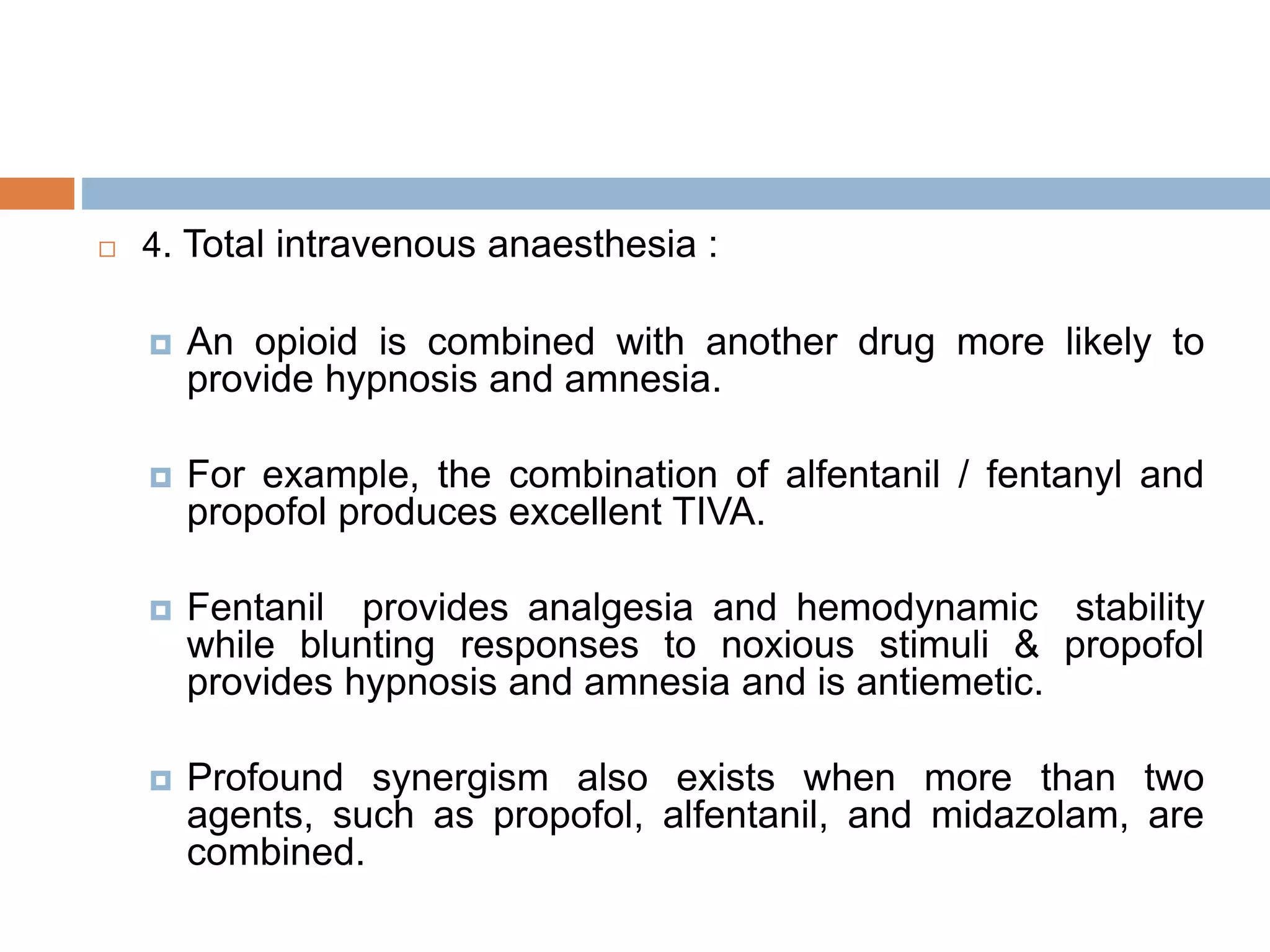
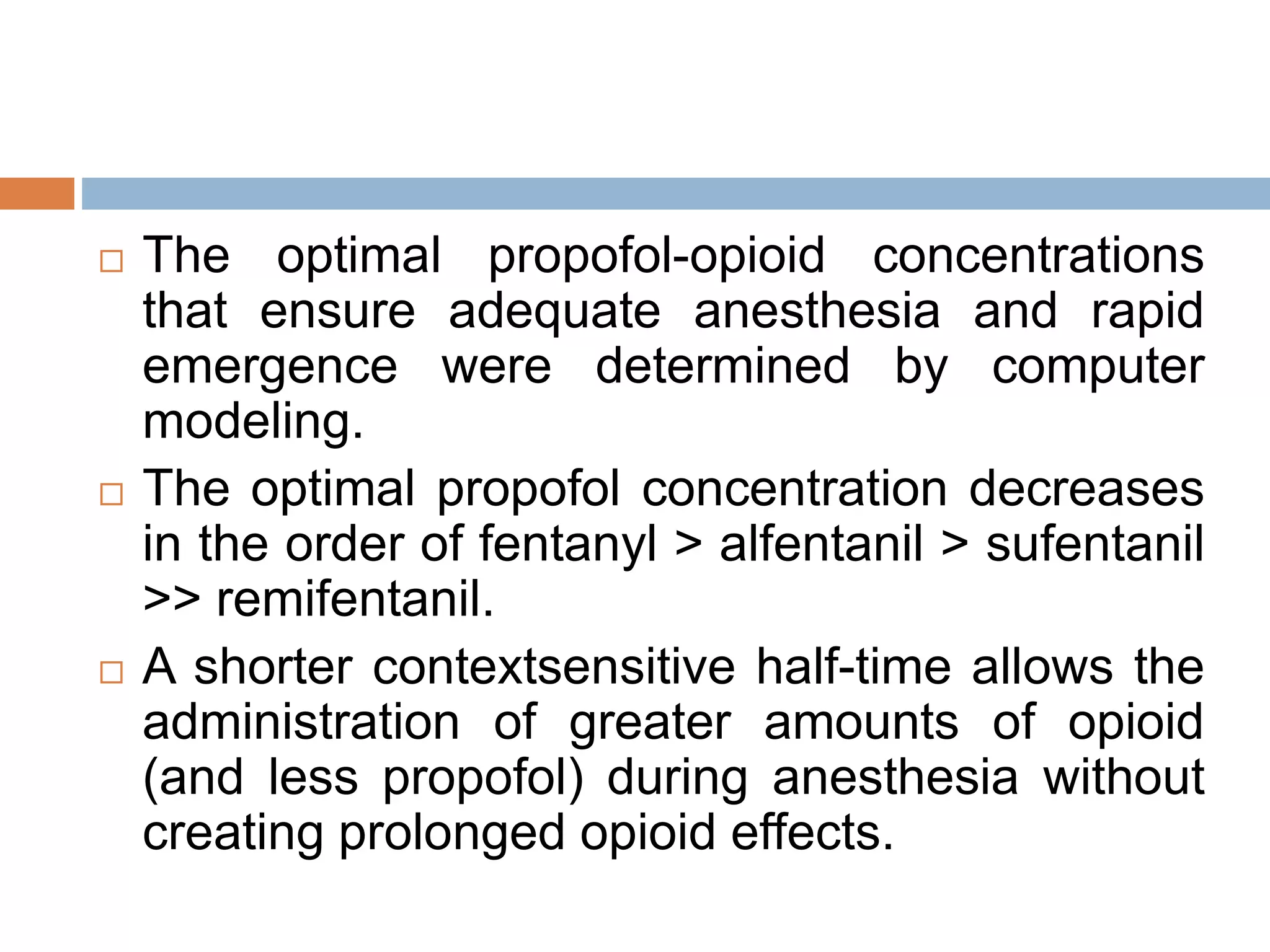
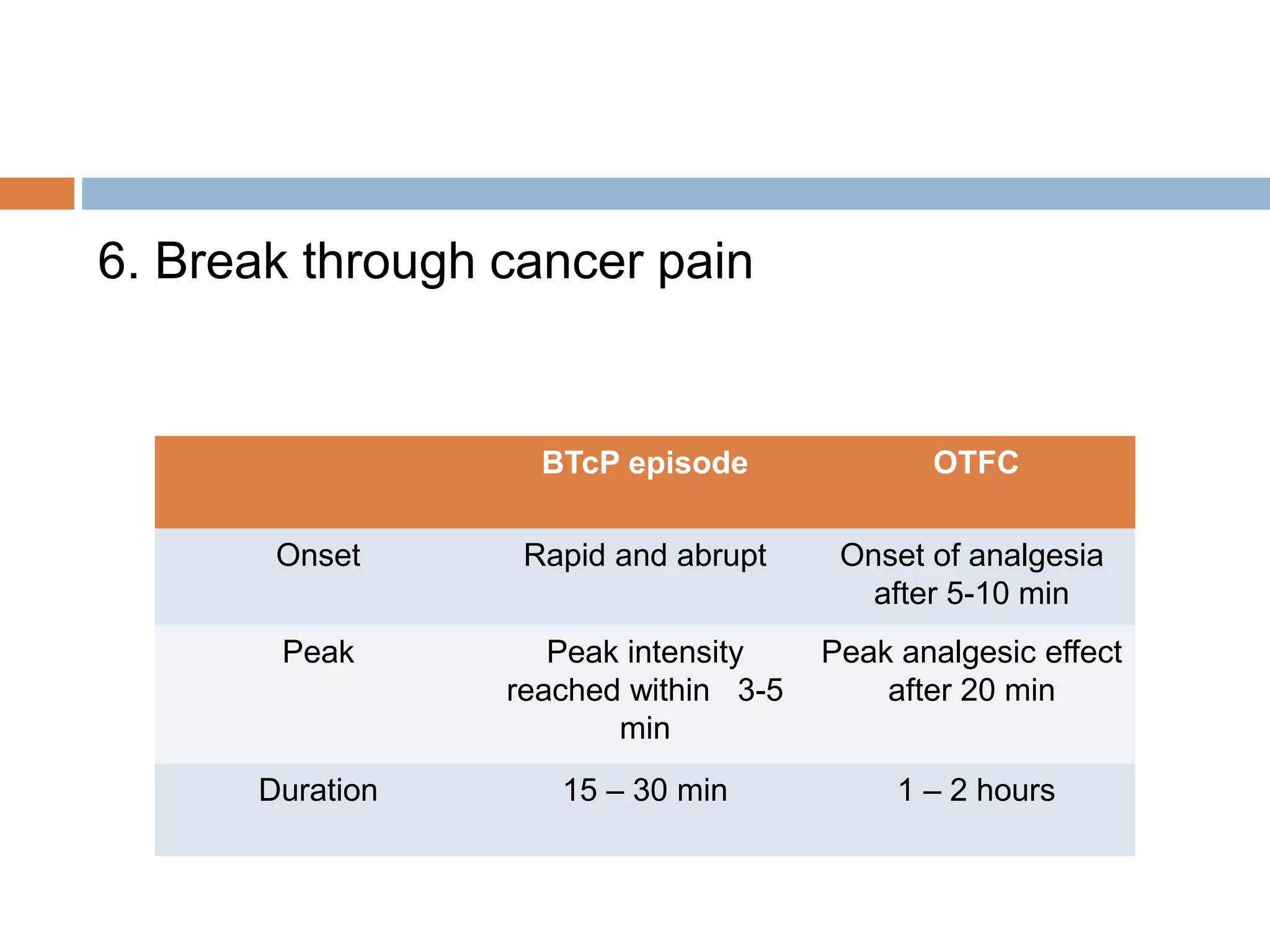
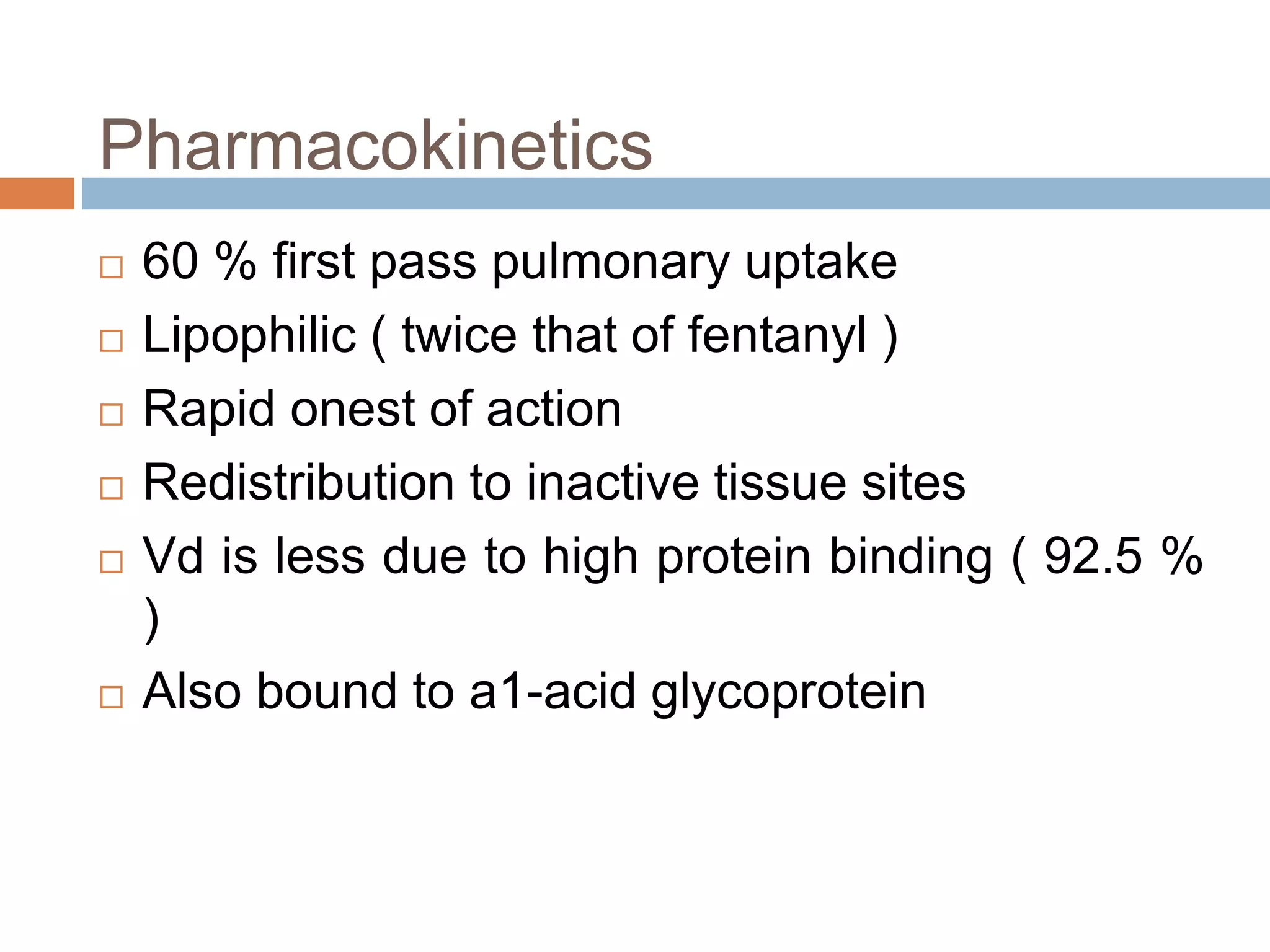
This document provides information on fentanyl and sufentanil, two synthetic opioids. It begins with an introduction to opioids in general and their classification. It then discusses opioid receptors and the mechanism of action of opioids like fentanyl and sufentanil. The pharmacokinetics, clinical uses, side effects and properties of fentanyl and sufentanil are described in detail. Fentanyl is noted to be 50-100 times more potent than morphine, while sufentanil is reported to be 10 times more potent than fentanyl. Both are useful for analgesia, anesthesia and managing acute or cancer pain.