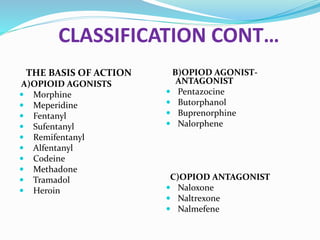
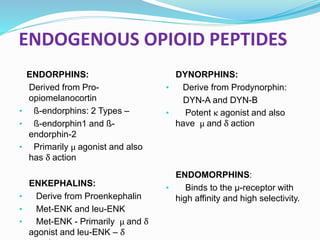
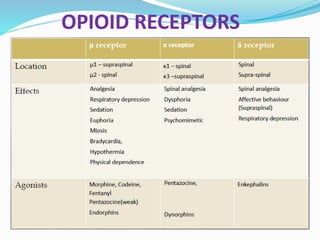
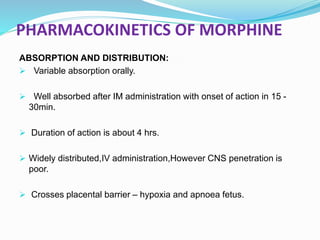
The document discusses various opioids including morphine, fentanyl, sufentanyl, meperidine, remifentanil, tramadol and pentazocine, outlining their history, classification, mechanisms of action, pharmacokinetics, clinical uses, and adverse effects. It provides details on the endogenous opioid peptides and opioid receptors in the body, as well as the therapeutic uses and pharmacology of morphine as the prototype opioid analgesic.