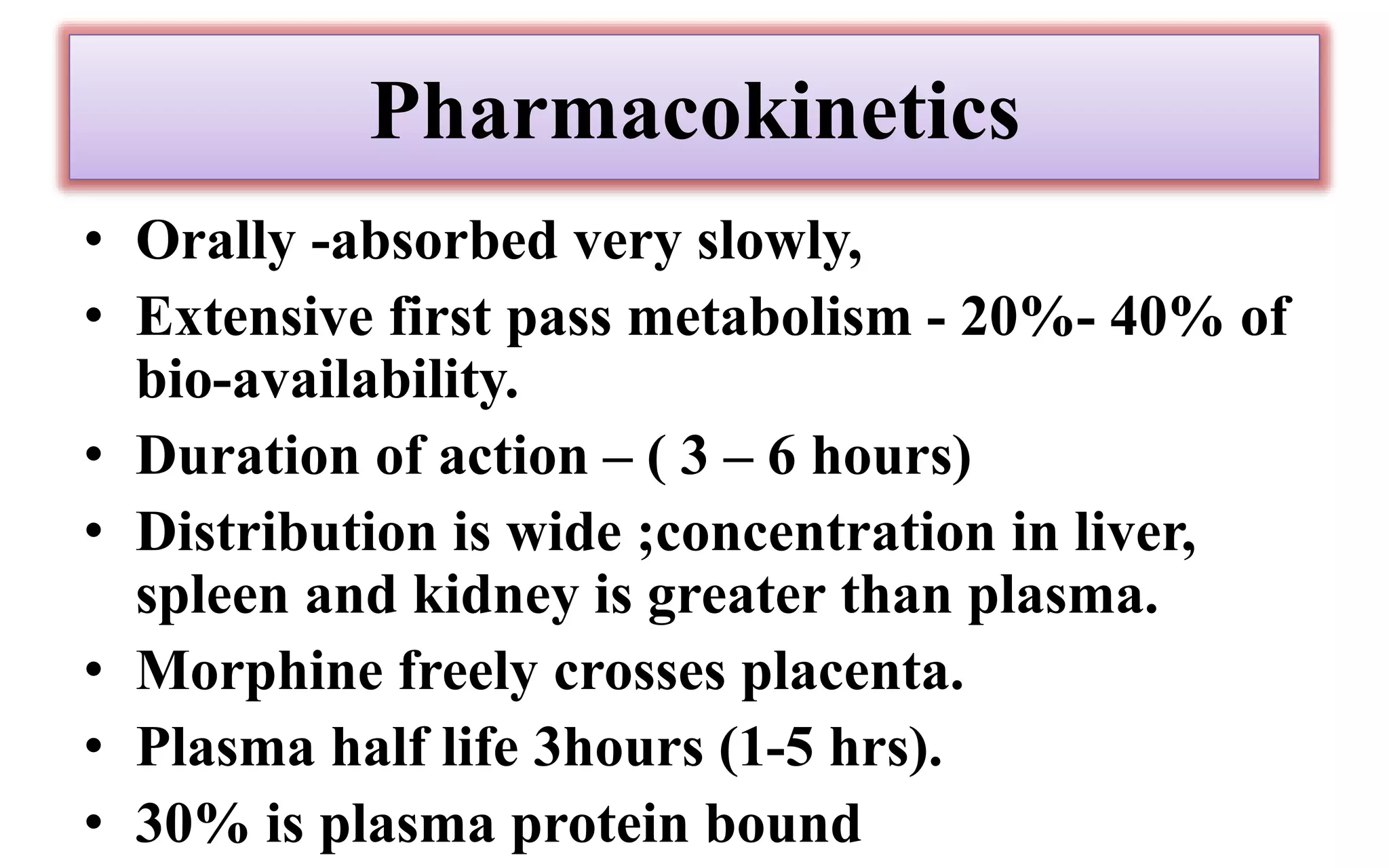
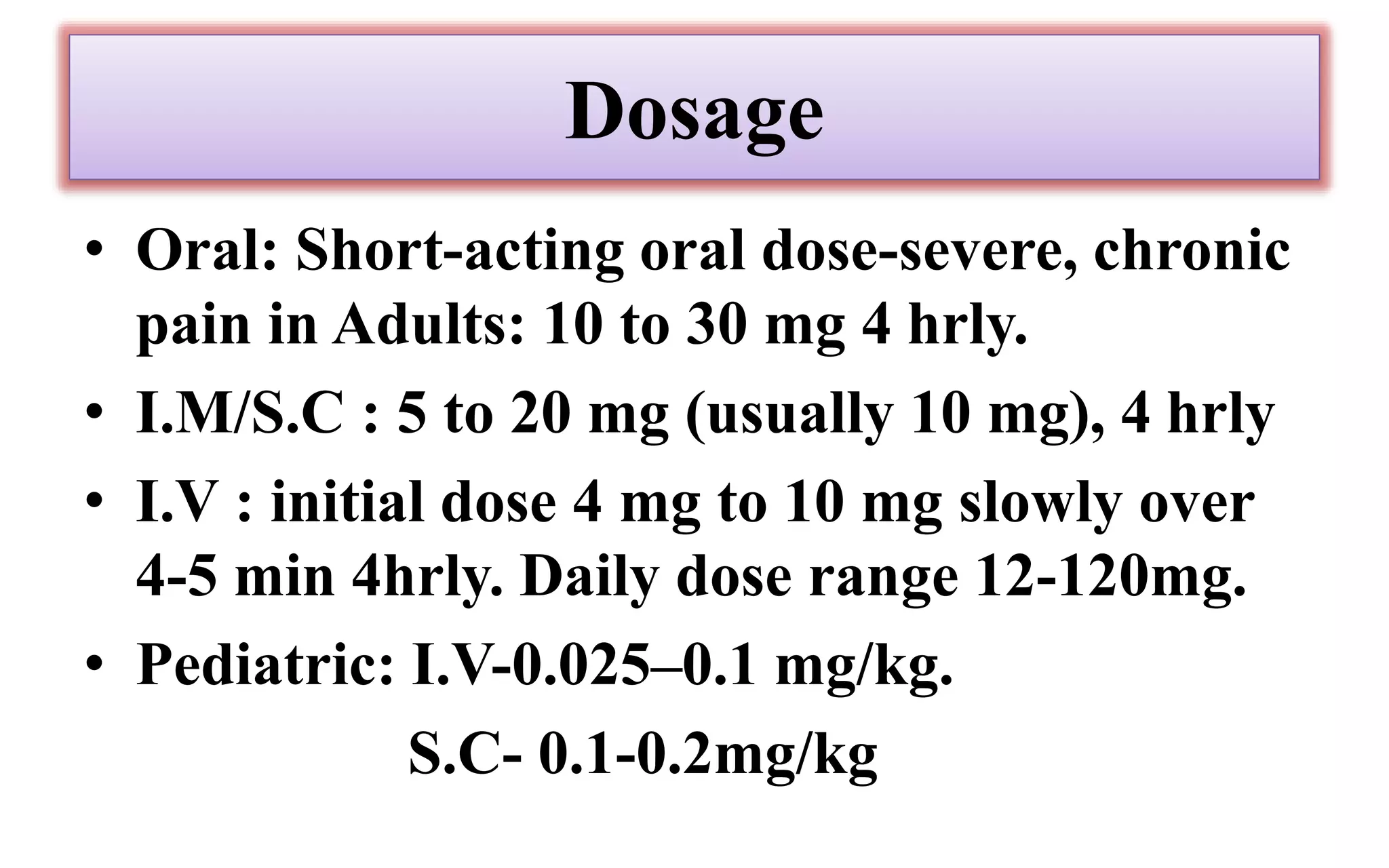
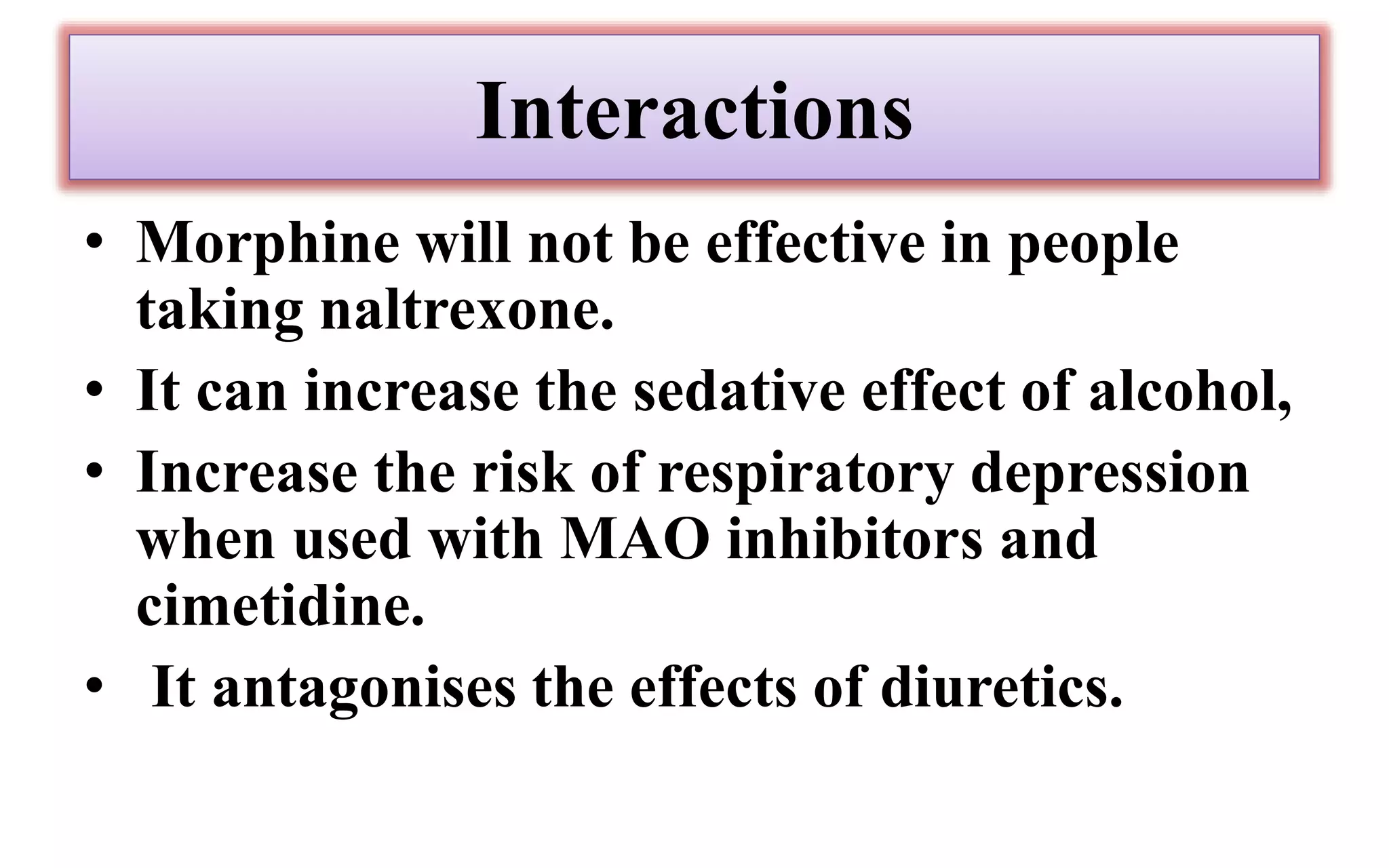
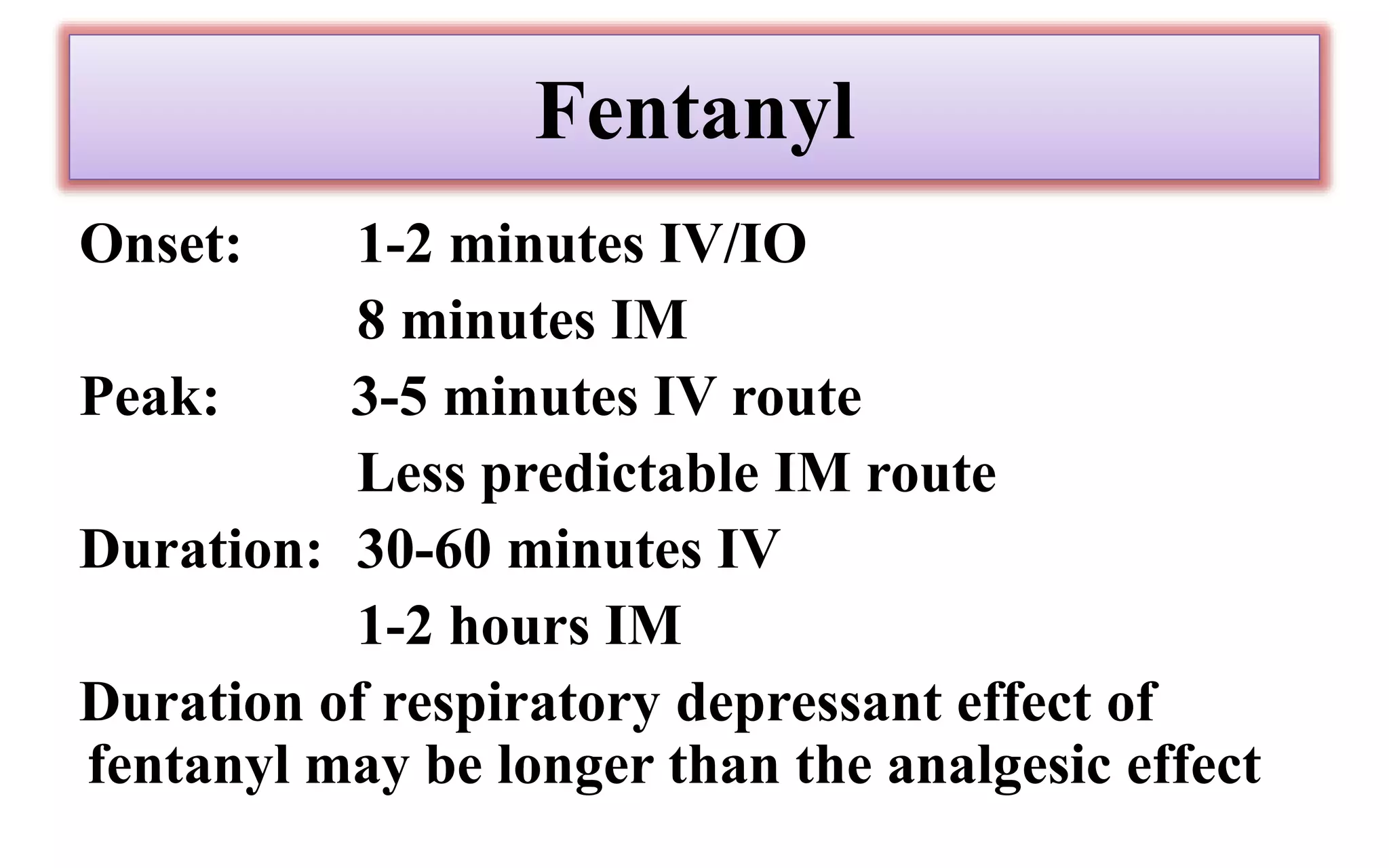
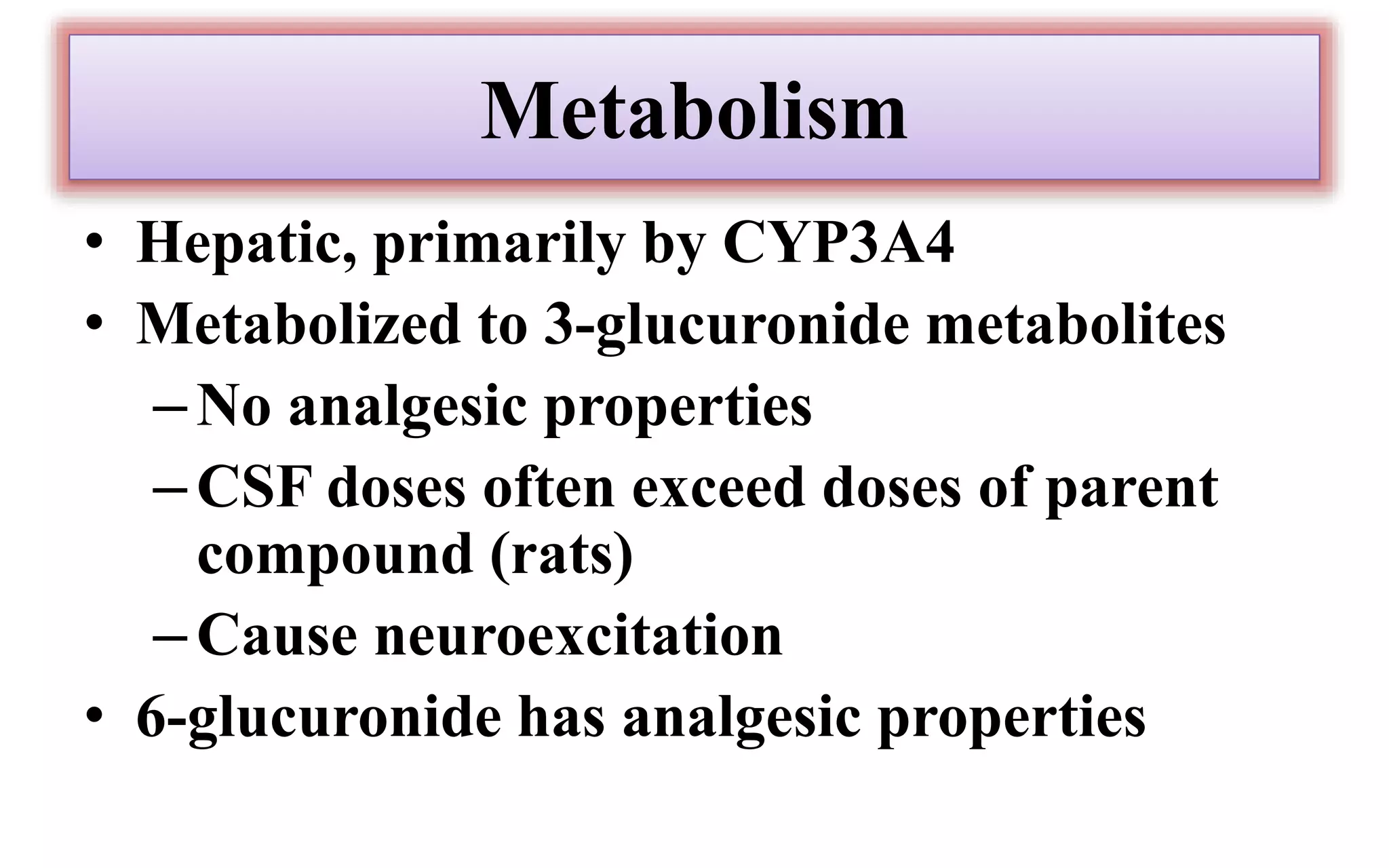
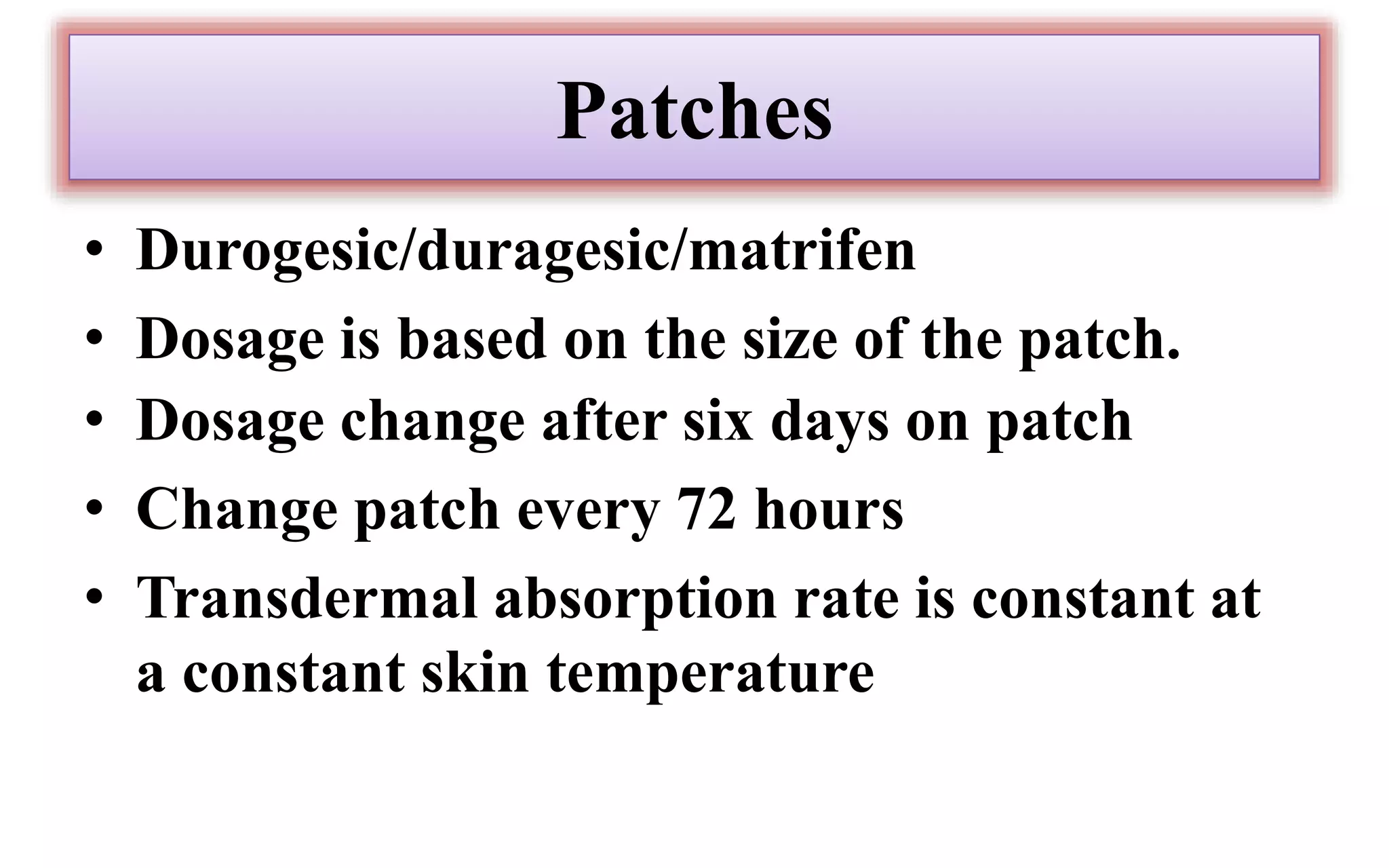
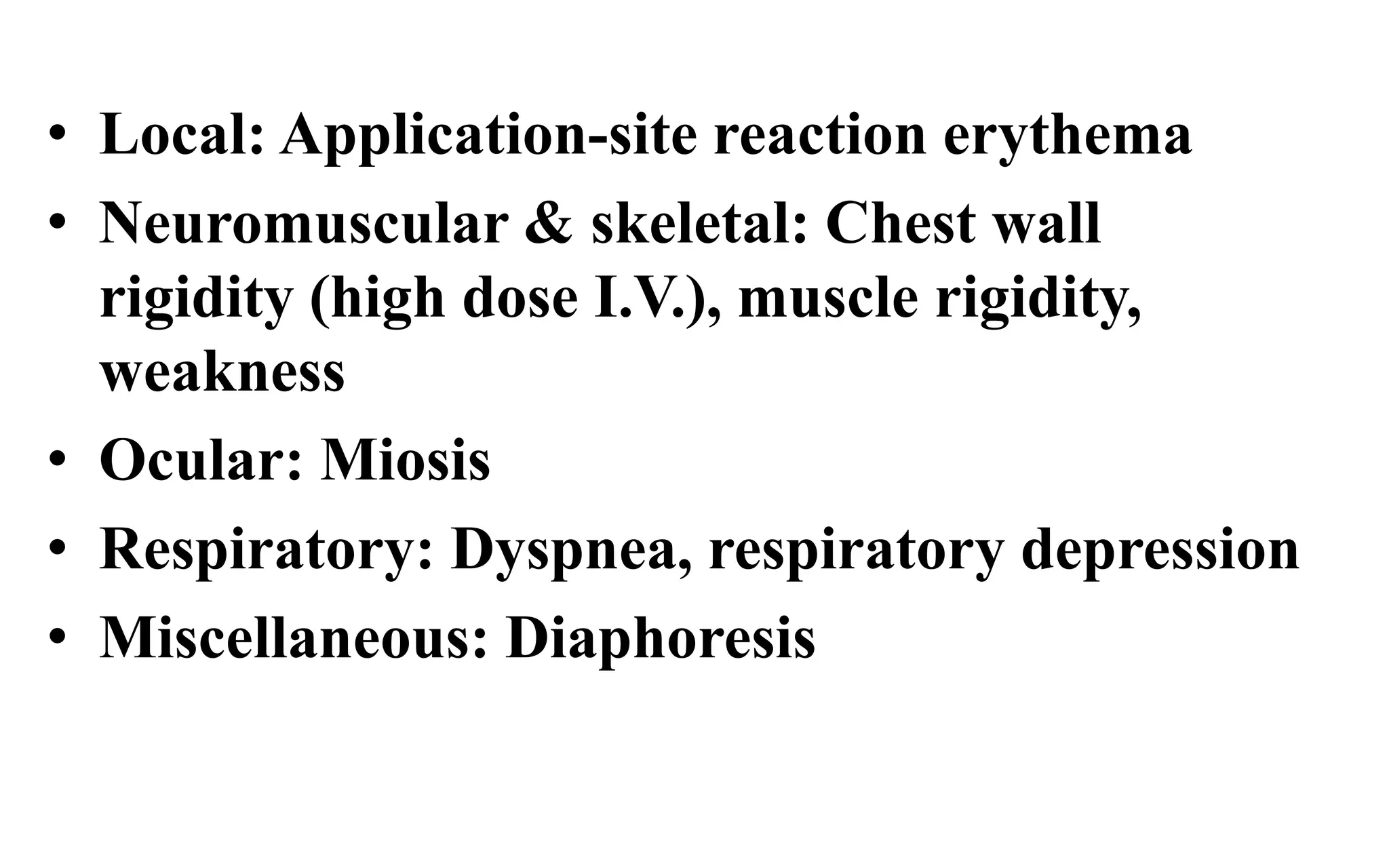
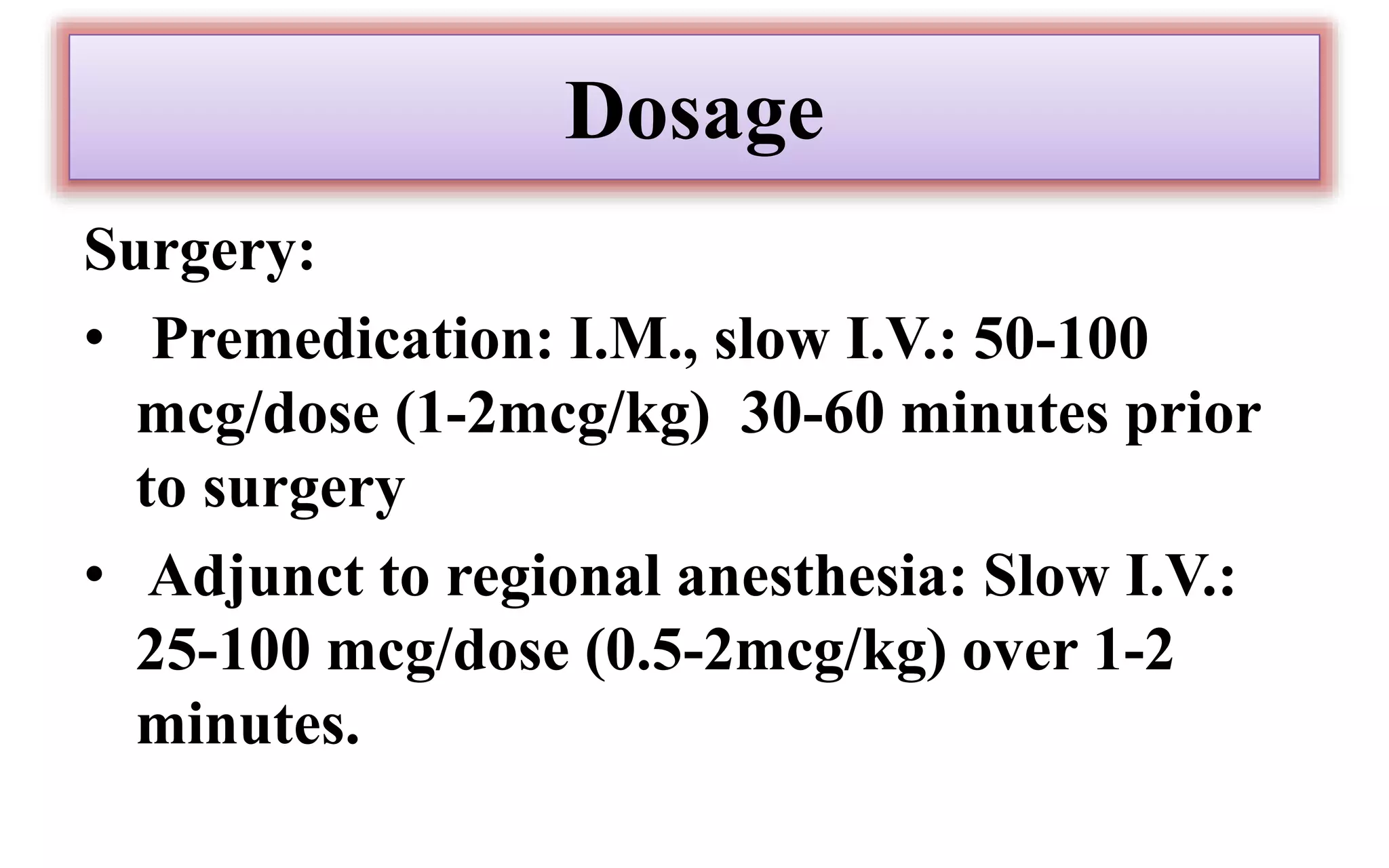
Morphine and fentanyl are potent opioid analgesics that act on mu-opioid receptors in the central nervous system. Morphine is a naturally occurring substance extracted from poppy plants, while fentanyl is a synthetic opioid. Both drugs are used medicinally to treat moderate to severe pain. Common side effects include respiratory depression, nausea, constipation, and euphoria. Withdrawal from long-term opioid use can cause pain, irritability, and dysphoria.