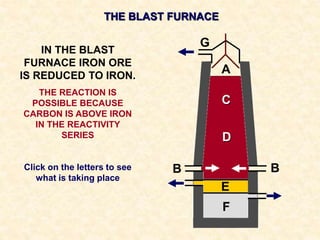
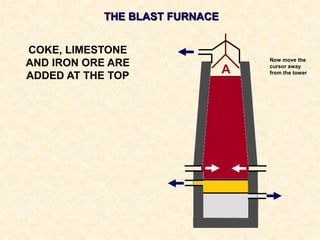
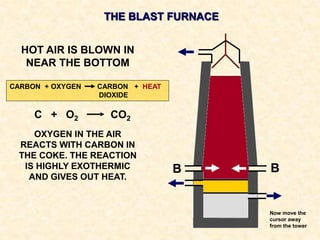
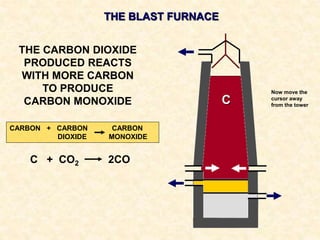
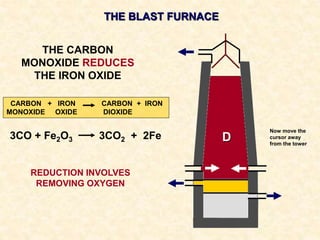
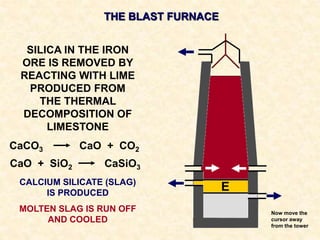
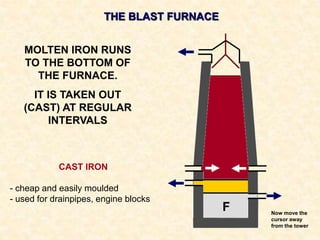
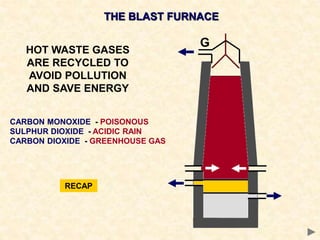
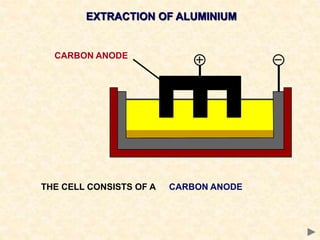
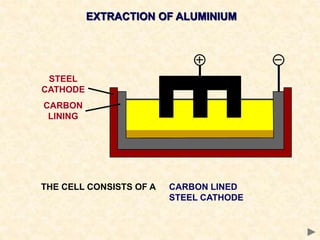
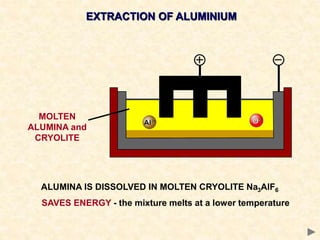
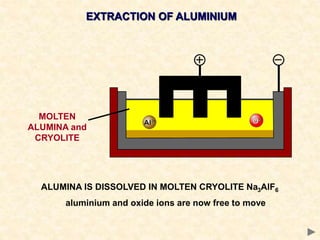
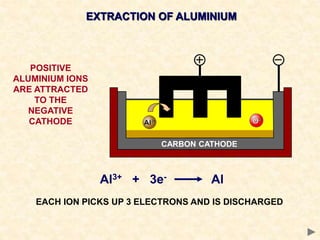
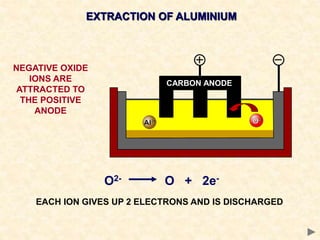
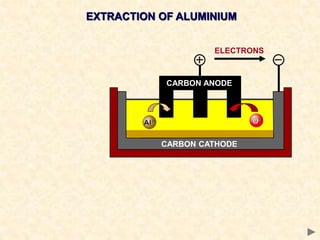
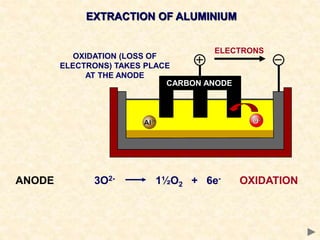
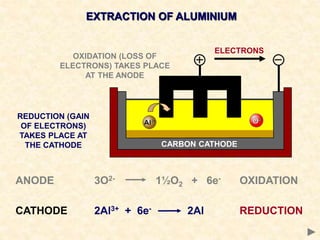
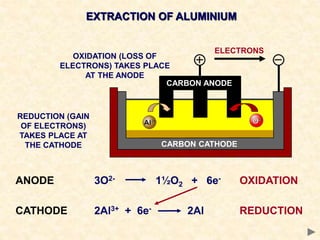
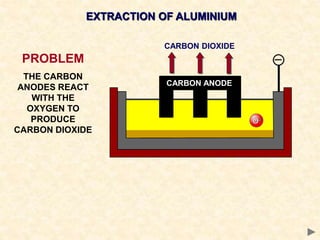
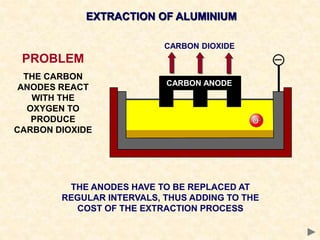
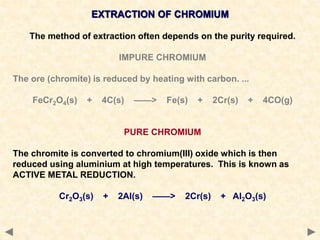
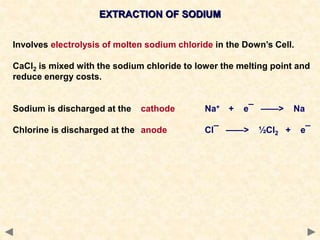
The document provides an overview of the extraction of metals. It discusses the extraction of iron through the blast furnace process using carbon reduction. Slag is produced to remove silica and waste gases can cause pollution. Steel is made from iron by removing excess carbon. The extraction of aluminium requires electrolysis due to its position in the reactivity series. Titanium extraction involves converting the oxide to the chloride then reducing with sodium.