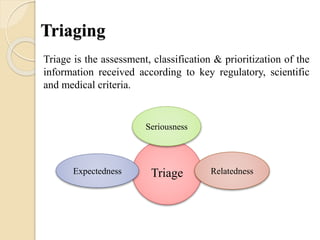
This document discusses the process of triaging adverse drug reaction (ADR) cases based on their seriousness and expectedness. It defines seriousness criteria such as death, life-threatening conditions, hospitalization, disability, and birth defects. It also discusses how to determine if a reported ADR is expected or unexpected based on a product's reference safety information. The key aspects of triaging covered are classifying ADR seriousness using FDA criteria, identifying verbatim or equivalent ADR terms in reference documents, considering other sections like warnings and precautions, and categorizing cases based on their combined seriousness and expectedness.