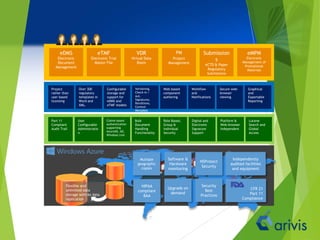
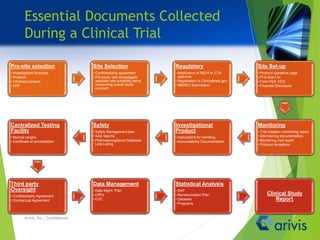
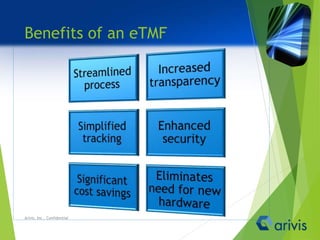
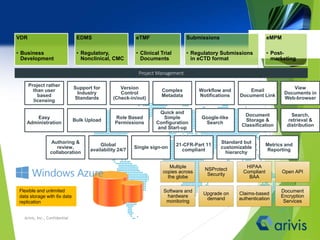
Arivis is a leader in regulatory information management, offering innovative cloud-based solutions for clinical and regulatory document management since 2005. Their flagship product, Clireo, supports electronic trial master files (eTMF), regulatory submissions, and project management, all compliant with FDA regulations and designed for cost-efficiency. Arivis also provides professional services to assist pharmaceutical and life science companies in organizing and managing their complex document processes efficiently.