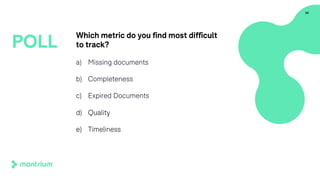
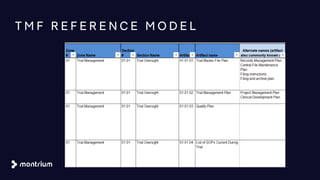
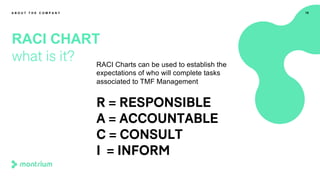
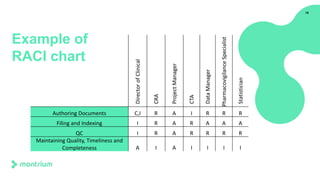
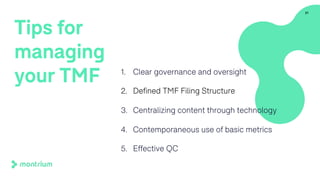
The document provides an overview of a webinar on TMF fundamentals presented by Montrium. The webinar agenda includes an introduction to Montrium, defining what a TMF is, common TMF challenges and how to alleviate them, leveraging regulations to ensure success, using basic metrics to better manage the TMF, and advantages of an electronic TMF. Key topics discussed include the TMF reference model, roles and responsibilities, tips for managing the TMF including clear governance and technology, and how an eTMF can help with collaboration, oversight, inspection readiness and metrics.























![30
Timeliness EMA:
“Article 57 states “the clinical trial master file shall at
all times contain the essential documents relating to
that clinical trial.” The requirement “at all times”
means that the TMF should be updated, and
completed in a timely manner.”
“[…] it is important, therefore, to keep the TMF up to
date, with documents placed in the TMF in a timely
manner as this greatly assists the successful
management of a trial by the investigator and
sponsor (or party to whom the sponsor has delegated
its duties).”
- ICH E6:
- “Filing essential documents at the
investigator/institution and sponsor sites in a timely
manner can greatly assist in the successful
management of a trial by the investigator, sponsor and
monitor.”](https://image.slidesharecdn.com/webinar-tmffundamentals-190321170212/85/Tmf-Fundamentals-webinar-30-320.jpg)