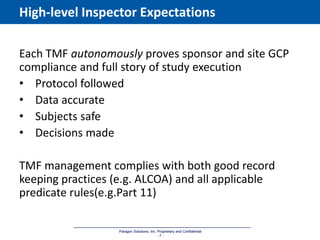
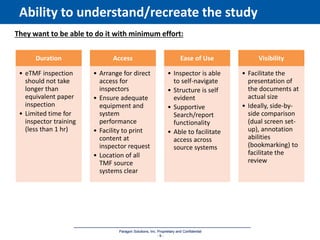
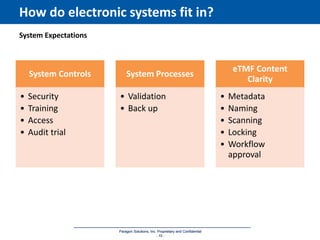
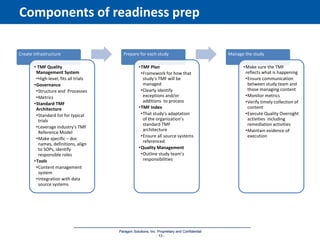
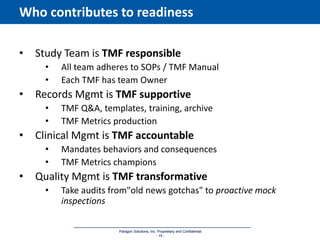
The document discusses the importance of Trial Master File (TMF) inspection readiness, outlining the need for continuous preparedness to meet Good Clinical Practice (GCP) standards and avoid negative consequences like failed inspections and increased costs. It highlights what inspectors expect from TMF management, including compliance, organization, and ease of access, while providing guidelines on how organizations can establish effective TMF systems. The document emphasizes that technology alone cannot ensure inspection readiness, but proper infrastructure, governance, and metric management can significantly enhance overall readiness.