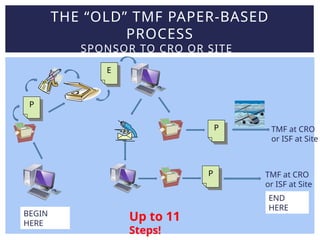
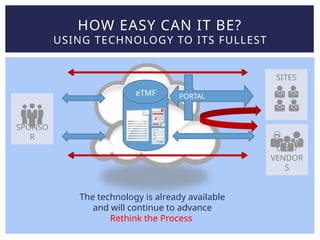
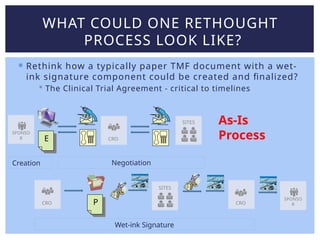
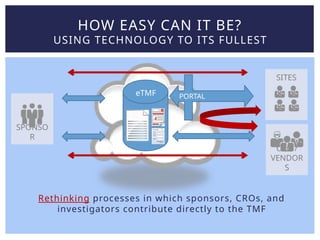
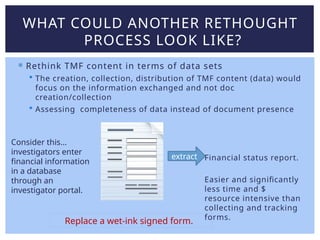
Lisa Mulcahy, an experienced consultant in the pharmaceutical research industry, focuses on improving trial master file (TMF) processes by leveraging technology for efficient data management and compliance. Her approach emphasizes the need to rethink traditional methods, such as reducing reliance on paper documents and signatures, to enhance collaboration among sponsors, CROs, and sites. Through strategic redesign of processes and utilization of electronic systems, Mulcahy aims to streamline TMF management and improve overall efficiency in clinical trials.