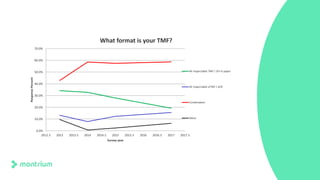
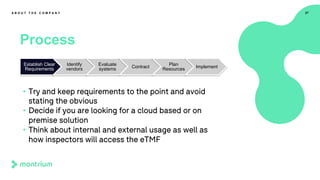
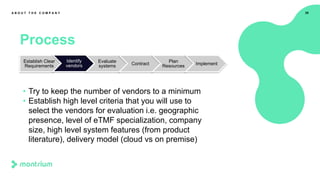
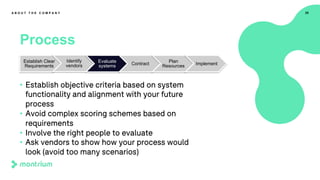
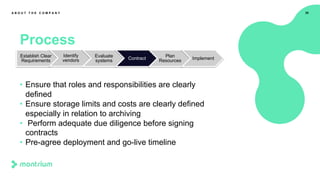
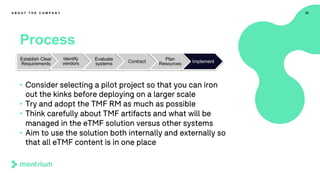
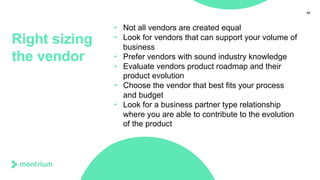
This webinar presentation provided an overview of practical steps for selecting and implementing an electronic trial master file (eTMF). It began with an introduction and housekeeping items. It then covered the changing regulatory landscape around eTMF requirements. Key challenges and the future of eTMF were discussed. The presentation outlined the process for eTMF vendor selection and provided tips for a successful implementation. It emphasized the importance of planning, change management, and adopting the TMF Reference Model.