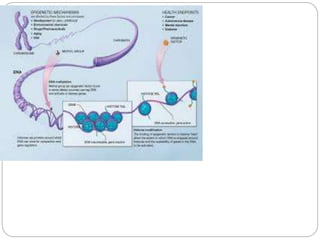
Epigenetics is the study of heritable changes in gene expression that are not caused by changes in DNA sequence. These changes are caused by mechanisms such as DNA methylation and histone modification. Epigenetics can modify gene activation and expression without changing the underlying DNA sequence. Epigenetic modifications like DNA methylation and chromatin remodeling are important in development and cell differentiation, allowing cells to have different functions while containing the same genetic information. Aberrant epigenetic changes are also involved in diseases like cancer, where genes can be inappropriately silenced through hypermethylation or other epigenetic alterations.