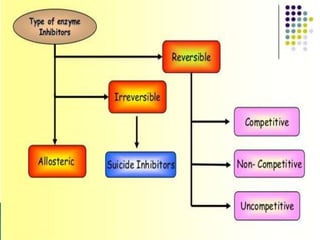
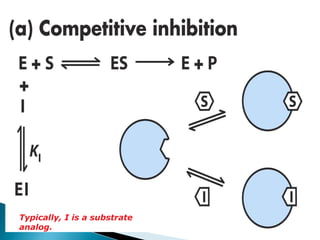
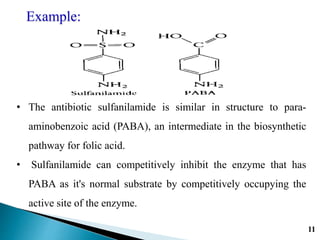
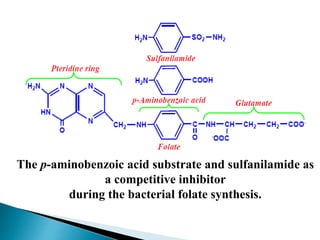
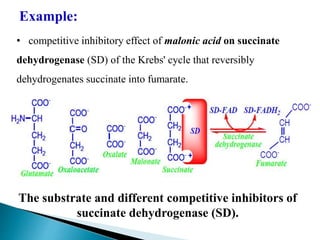
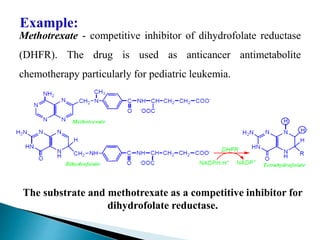
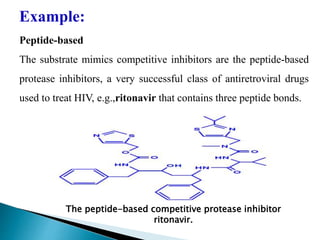
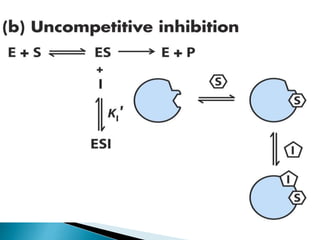
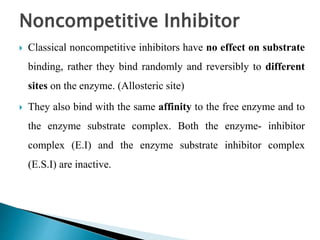
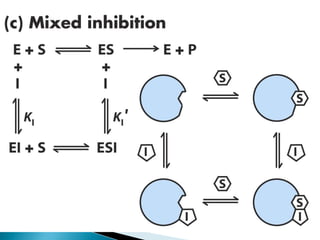
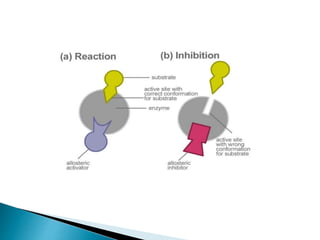
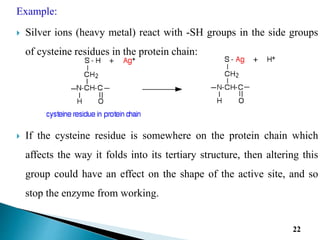
Reversible enzyme inhibitors can reduce or inhibit enzyme catalytic activity through reversible, non-covalent interactions with the enzyme. There are three main types of reversible inhibitors: competitive inhibitors, which compete with the substrate for the active site; uncompetitive inhibitors, which only bind to the enzyme-substrate complex; and noncompetitive inhibitors, which bind randomly to the free enzyme or enzyme-substrate complex, inhibiting both. Reversible inhibitors form equilibrium complexes with the enzyme and do not permanently modify it, allowing activity to be restored once the inhibitor is removed. Examples of reversible inhibitors discussed include sulfanilamide as a competitive inhibitor of PABA and methotrexate as a competitive inhibitor of dihydrofolate reductase.



![Michaelis-menten equation
The michaelis-menten equation arises from the
general equation for an enzymatic reaction.
E+S ES E+P
The michaelis menten equation is:
V˳=
Where=
V˳= velocity of the reaction
Vmax= maximal rate of the reaction
[substrate]= conc. Of the substrate
Km= michaelis-menten constant
Vmax [S]
km+[S]
6](https://image.slidesharecdn.com/evalvationsaminar-171121162144/85/Enzyme-Inhibitors-6-320.jpg)