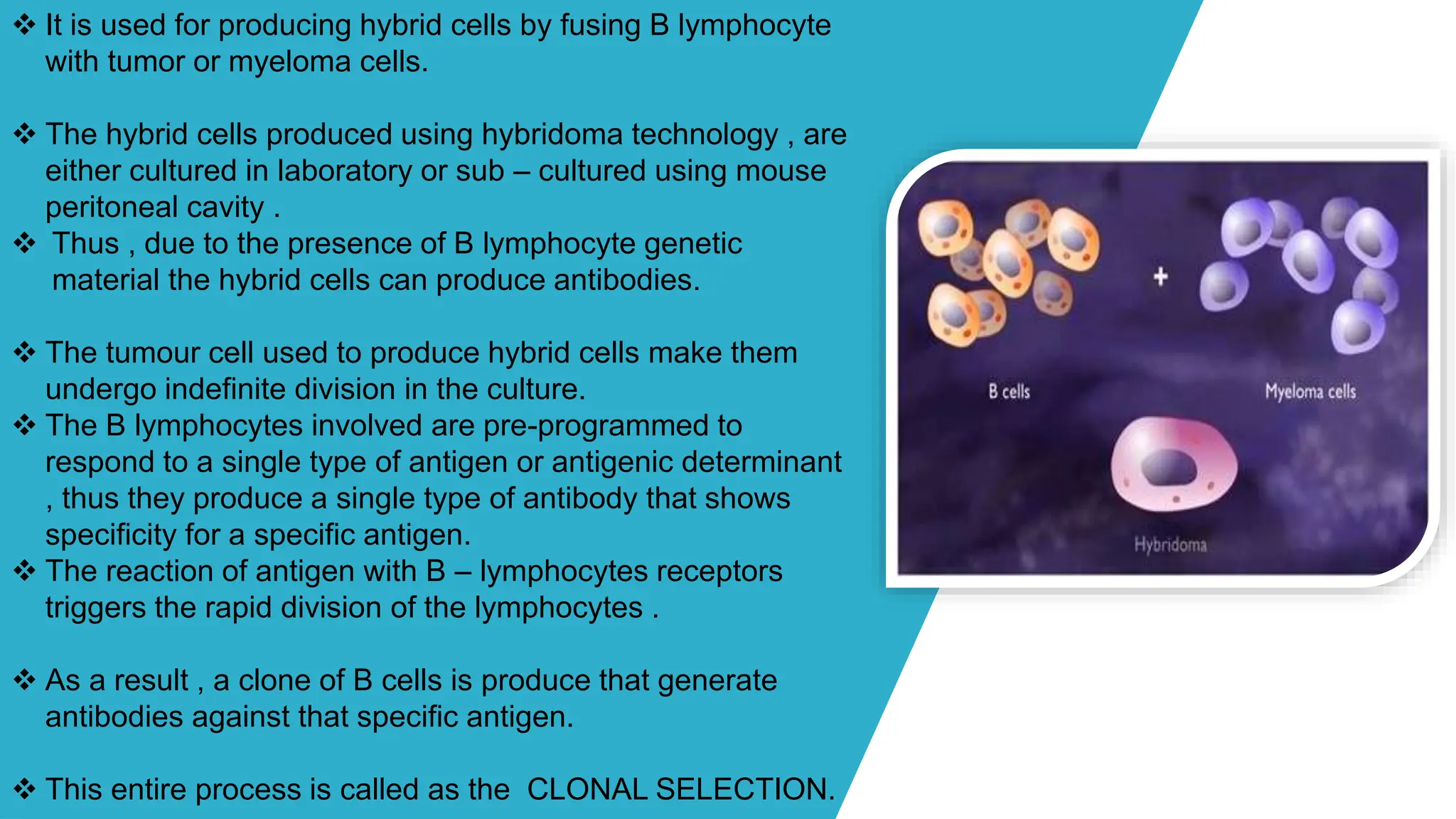
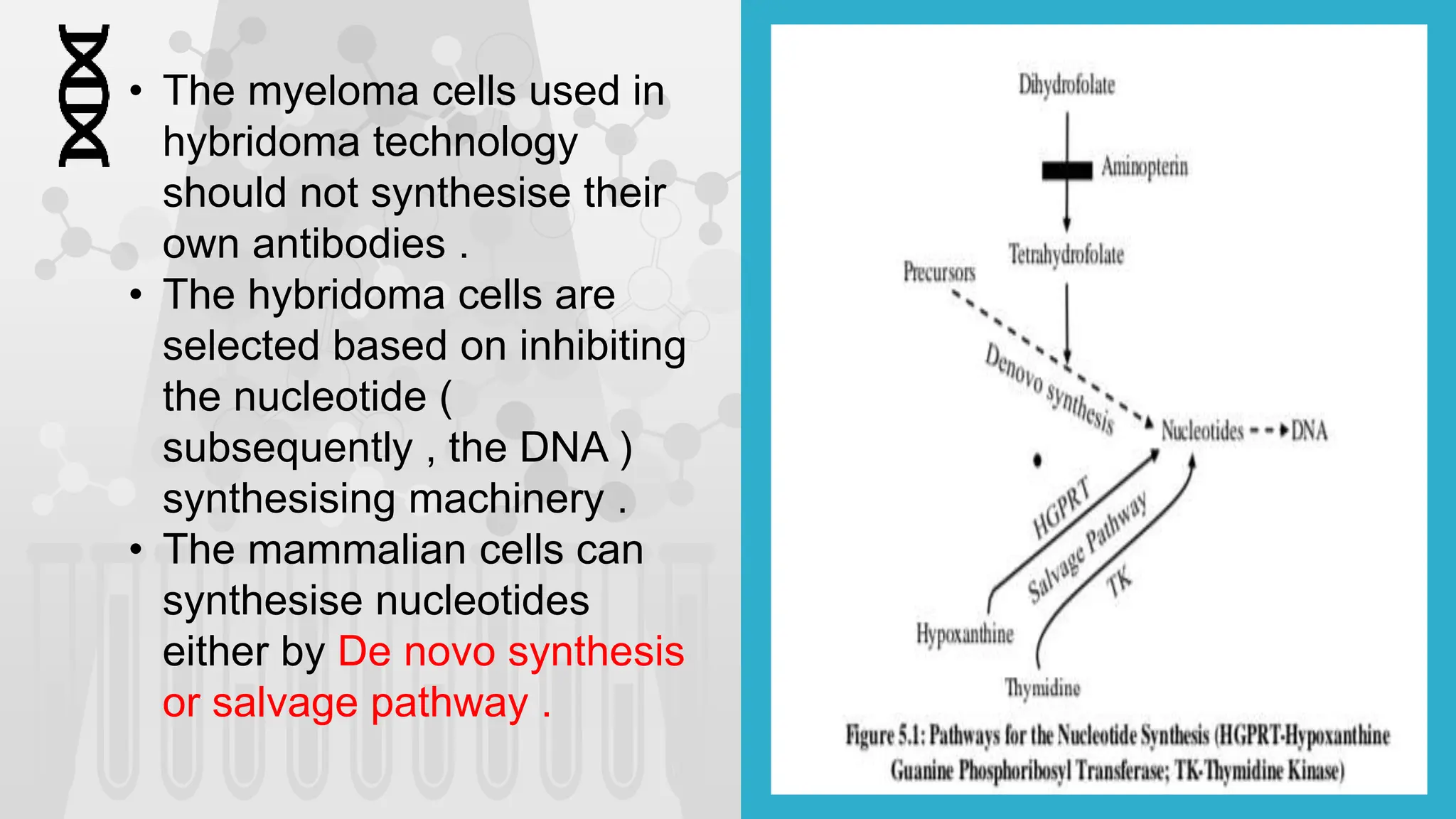
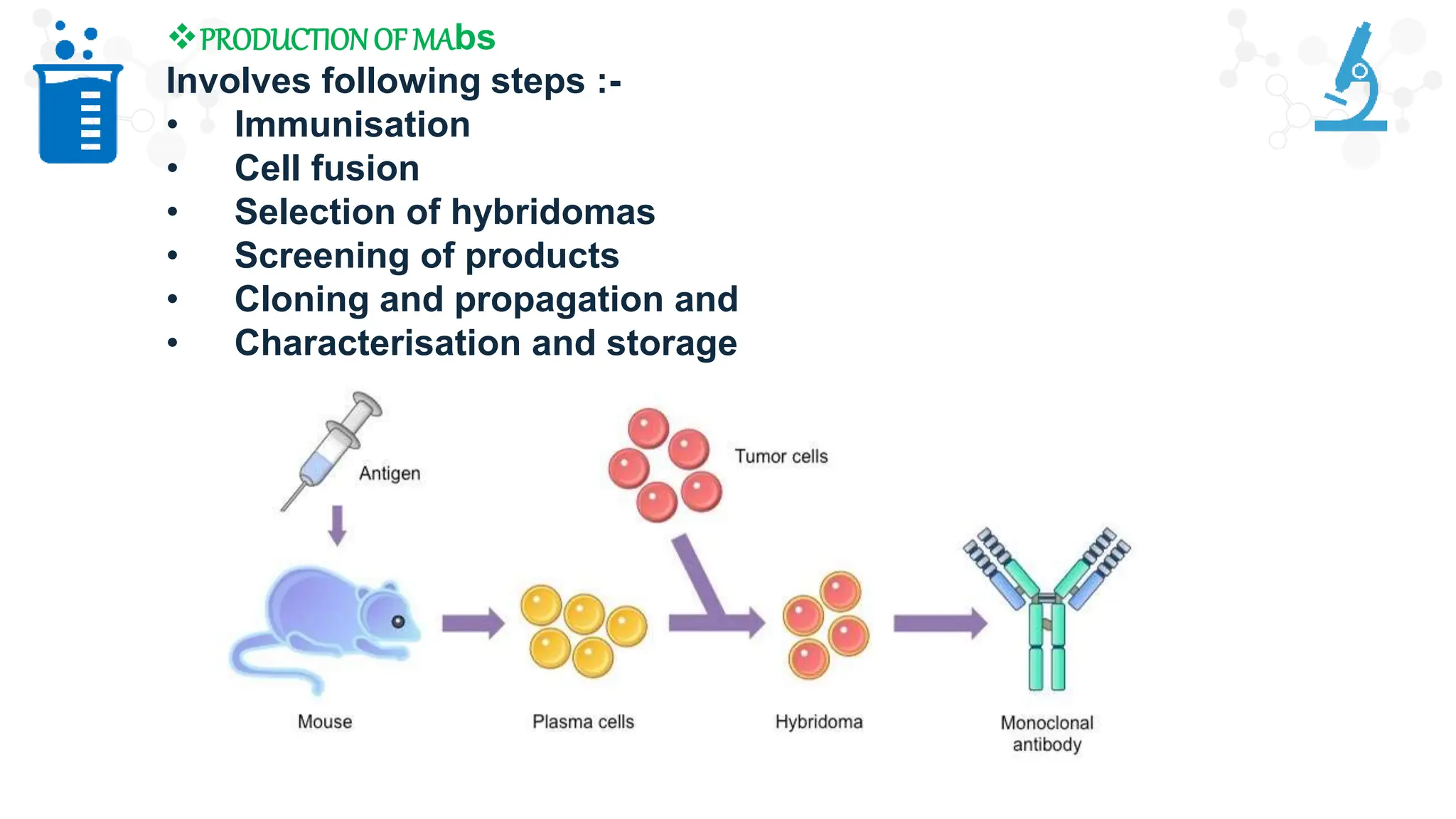
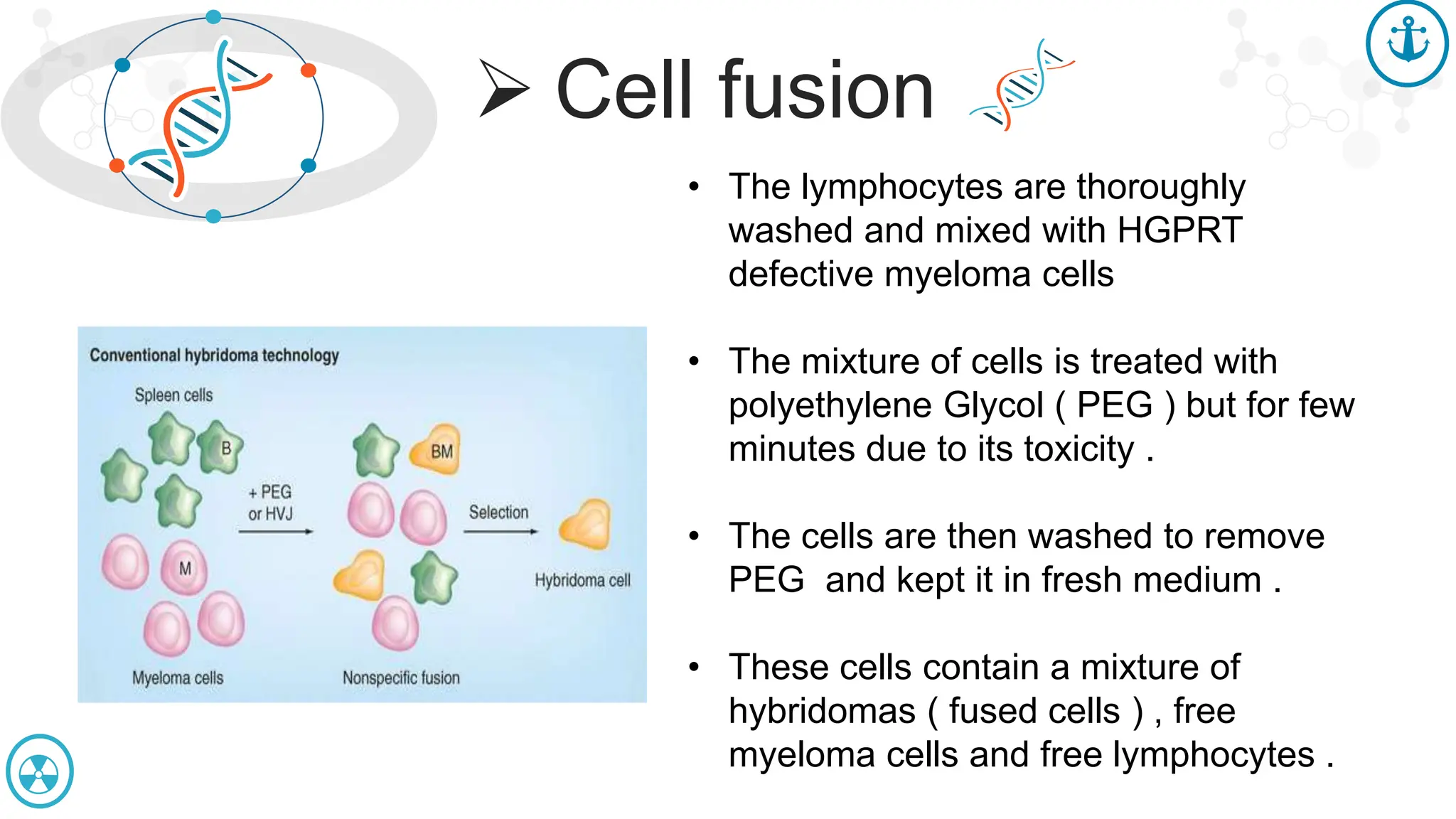
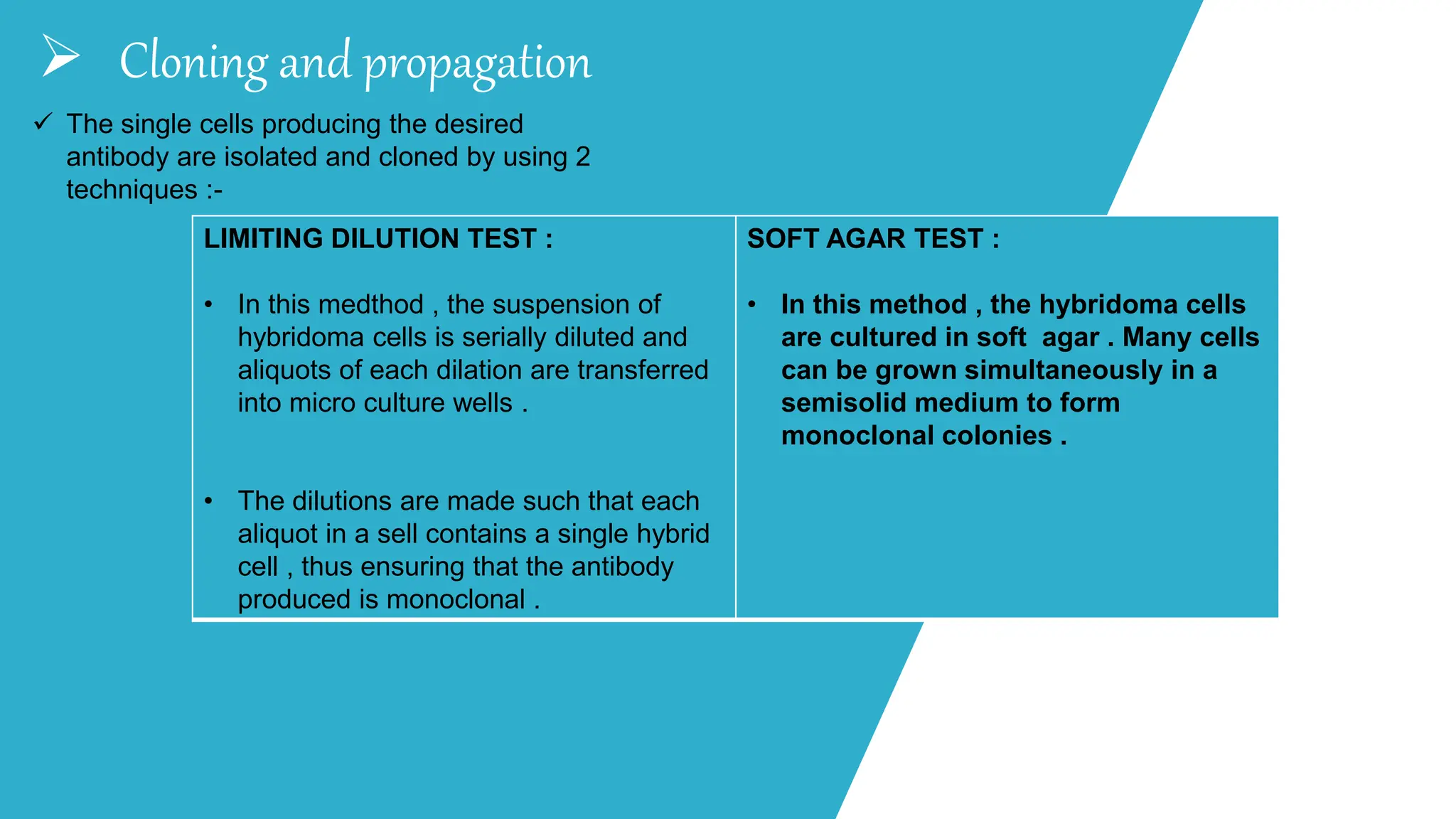
The document discusses hybridoma technology, which was developed in 1975 for the production of monoclonal antibodies (mAbs) by fusing B lymphocytes with myeloma cells to create hybrid cells that can produce specific antibodies. It outlines the advantages of monoclonal antibodies over polyclonal ones, the steps involved in their production, purification methods, and various diagnostic and therapeutic applications. Additionally, the document details techniques for selection, screening, cloning, and characterization of hybridoma cells and mAbs.