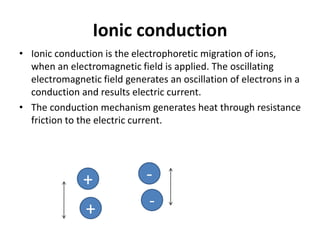
The document discusses microwave-assisted reactions in chemistry, highlighting their advantages over conventional heating, such as rapid and homogeneous heating that leads to higher yields and fewer side-products. It describes various mechanisms of microwave heating and types of reactions, including dry media and solvent-mediated synthesis, emphasizing the efficiency and purity of products obtained. Additionally, it addresses applications in organic synthesis and the food industry while noting some disadvantages, such as risks associated with sudden temperature increases.