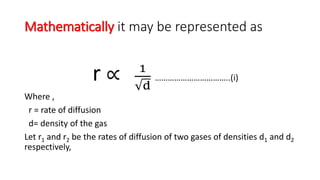
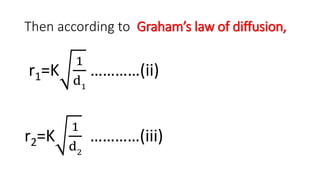
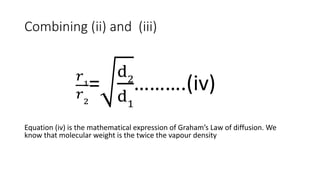
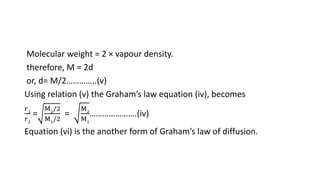
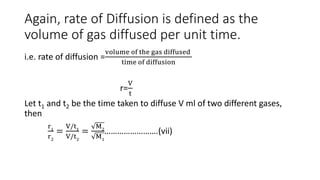
Graham's Law of Diffusion states that the rates of diffusion of gases are inversely proportional to the square root of their densities. Under similar conditions of temperature and pressure, lighter gases will diffuse faster than heavier gases. The time taken for equal volumes of different gases to diffuse is directly proportional to the square roots of their molecular weights or densities. Graham's Law helps to separate gases based on differences in their densities and determine properties of unknown gases.