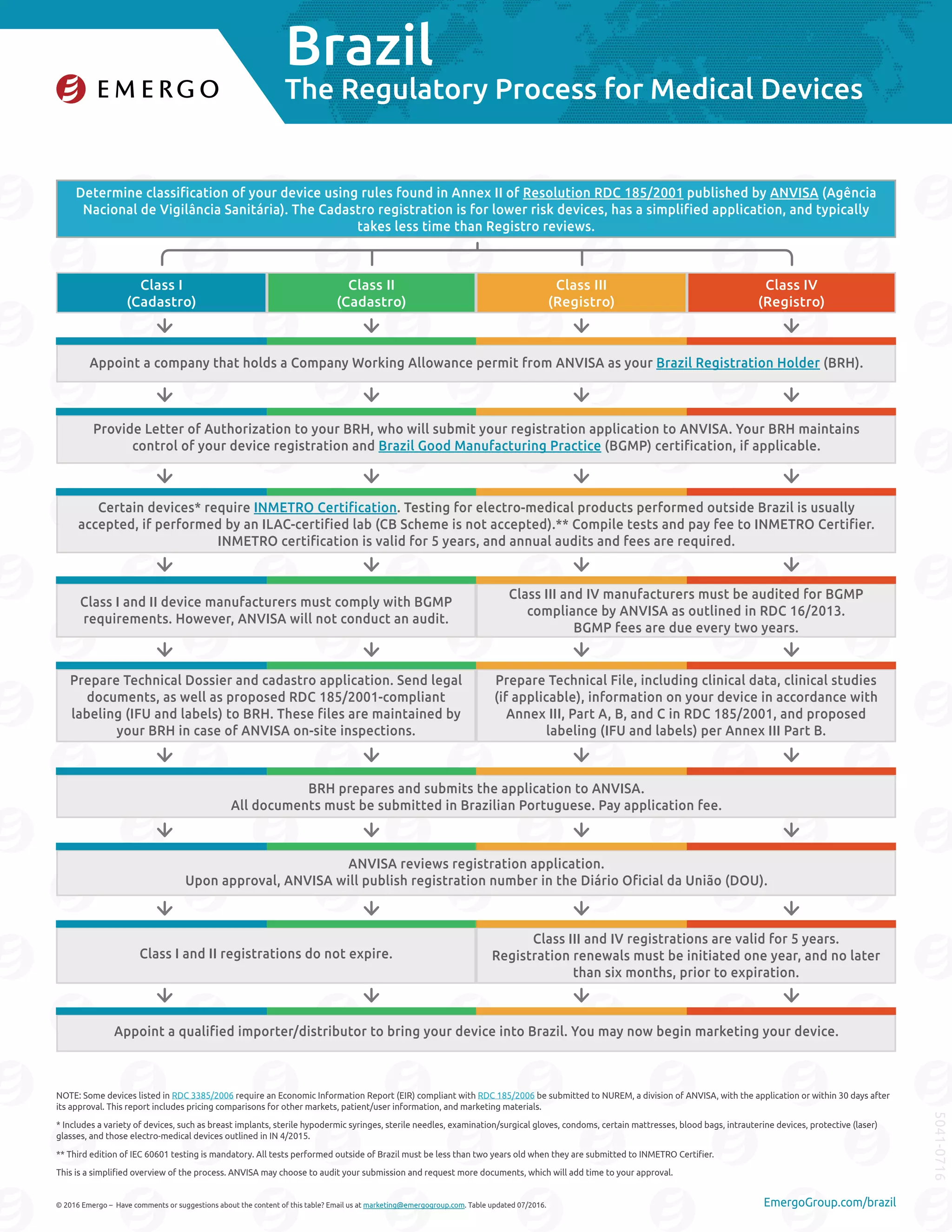
Brazil has a classification system for medical devices with four classes - I, II, III, and IV. Class I and II devices require a simplified registration called a Cadastro, while Class III and IV devices require a more rigorous registration called a Registro. To register a device, a company must appoint a Brazil Registration Holder to submit the application to ANVISA and obtain necessary approvals. Certain devices may also require certification from inspection agency INMETRO. The registration process can take 1-3 months for Classes I and II or 8-15 months for Classes III and IV, though Class III and IV can take over 4 years if additional inspections are required.