Japan medical device approval chart - Emergo
•
5 likes•4,614 views
The regulatory process for medical devices in Japan involves several key steps: 1) Classifying the device and appointing a Marketing Authorization Holder who will manage the registration process. 2) Implementing a Quality Management System that complies with relevant regulations. 3) Submitting an application to either the Pharmaceuticals and Medical Devices Agency or a Registered Certification Body, depending on the device class. 4) Undergoing an audit and receiving the appropriate certificate before marketing the device in Japan. Device registrations do not expire but must be renewed periodically.
Report
Share
Report
Share
Download to read offline
Recommended
Taiwan medical device registration and approval chart - EMERGO

Taiwan regulates medical devices through the Pharmaceutical Affairs Act and Regulations for Governing the Management of Medical Devices. The process involves classifying the device, appointing a Taiwan agent, preparing quality system documentation for submission, and obtaining approval. Device classification and complexity of approval requirements vary, with Class I generally having the simplest process taking 1-2 months, Class II taking 10-12 months, and Class III taking 10-12 months and requiring a committee review.
Brazil medical device registration and approval chart - EMERGO

Brazil has a classification system for medical devices with four classes - I, II, III, and IV. Class I and II devices require a simplified registration called a Cadastro, while Class III and IV devices require a more rigorous registration called a Registro. To register a device, a company must appoint a Brazil Registration Holder to submit the application to ANVISA and obtain necessary approvals. Certain devices may also require certification from inspection agency INMETRO. The registration process can take 1-3 months for Classes I and II or 8-15 months for Classes III and IV, though Class III and IV can take over 4 years if additional inspections are required.
China medical device approval chart - EMERGO

1. The document outlines the regulatory process for medical devices in China, including classification of devices and the approval process for Class I, II, and III devices.
2. Class I devices have the simplest approval process, requiring only an administrative review with no submission fees. Class II and III devices require more extensive technical documentation, testing in China, and clinical evaluations.
3. The approval process can take 12-22 months for Class II and III devices and involves appointing a Chinese agent, submitting documentation and application materials in Chinese, testing, and a review by the China Food and Drug Administration before a registration certificate is issued.
Medical device approval chart for Russia - EMERGO

The regulatory process for medical devices in Russia involves classifying the device, appointing an authorized representative, submitting a registration dossier including technical documentation and clinical data to Roszdravnadzor (RZN), addressing any requests for additional information, and ultimately receiving a registration certificate to market the device in Russia. The process can take 6-12 months depending on the device class.
Canada medical device approval chart - EMERGO

1. To market medical devices in Canada, manufacturers must classify their device according to regulations, implement an ISO 13485 quality management system with additional Canadian requirements, and obtain ISO 13485 certification from an accredited registrar.
2. Manufacturers then prepare license applications, supporting documents, and pay fees, with process times ranging from 1-8 months depending on device class.
3. Licenses must be renewed annually by meeting regulatory requirements and paying fees to avoid revocation.
India medical device regulatory process

The regulatory framework for medical devices in India is based on drug regulations under the Drugs and Cosmetics Act of 1940 and Drugs and Cosmetics Rules of 1945. The Drug Controller General of India (DCGI) within the Central Drugs Standard Control Organization (CDSCO) regulates medical devices and IVDs. Currently only a limited number of medical device and IVD products require registration in India, including ablation devices, dental implants, and hernia mesh. The registration process for notified devices can take 9-12 months and involves appointing an authorized agent, compiling an application, and obtaining CDSCO approval.
Medical device approval chart for Mexico - Emergo 

In Mexico, medical devices and in-vitro diagnostic devices are regulated by COFEPRIS, a division of the Mexican Ministry of Health. The regulatory process involves classifying the device, appointing a Mexico Registration Holder representative, preparing a registration dossier in Spanish including documentation of quality management and technical specifications, and submitting the application to COFEPRIS for review. Approval time ranges from 1-10 months depending on the device class and review process. Registrations are valid for 5 years and must be renewed in advance of the expiration date.
Europe IVD medical registration and approval chart - EMERGO

The document summarizes the regulatory process for in vitro diagnostic devices (IVDs) in Europe under the In Vitro Diagnostic Directive (98/79/EC). It outlines the classification of IVDs, requirements for quality management systems and technical files, roles of notified bodies and authorized representatives, and timelines and costs associated with the approval process depending on the IVD classification. The process can take from 3-5 months for self-certified IVDs to 9-12 months for list A IVDs and involves implementing quality systems, obtaining notified body audits, and registering with authorities.
Recommended
Taiwan medical device registration and approval chart - EMERGO

Taiwan regulates medical devices through the Pharmaceutical Affairs Act and Regulations for Governing the Management of Medical Devices. The process involves classifying the device, appointing a Taiwan agent, preparing quality system documentation for submission, and obtaining approval. Device classification and complexity of approval requirements vary, with Class I generally having the simplest process taking 1-2 months, Class II taking 10-12 months, and Class III taking 10-12 months and requiring a committee review.
Brazil medical device registration and approval chart - EMERGO

Brazil has a classification system for medical devices with four classes - I, II, III, and IV. Class I and II devices require a simplified registration called a Cadastro, while Class III and IV devices require a more rigorous registration called a Registro. To register a device, a company must appoint a Brazil Registration Holder to submit the application to ANVISA and obtain necessary approvals. Certain devices may also require certification from inspection agency INMETRO. The registration process can take 1-3 months for Classes I and II or 8-15 months for Classes III and IV, though Class III and IV can take over 4 years if additional inspections are required.
China medical device approval chart - EMERGO

1. The document outlines the regulatory process for medical devices in China, including classification of devices and the approval process for Class I, II, and III devices.
2. Class I devices have the simplest approval process, requiring only an administrative review with no submission fees. Class II and III devices require more extensive technical documentation, testing in China, and clinical evaluations.
3. The approval process can take 12-22 months for Class II and III devices and involves appointing a Chinese agent, submitting documentation and application materials in Chinese, testing, and a review by the China Food and Drug Administration before a registration certificate is issued.
Medical device approval chart for Russia - EMERGO

The regulatory process for medical devices in Russia involves classifying the device, appointing an authorized representative, submitting a registration dossier including technical documentation and clinical data to Roszdravnadzor (RZN), addressing any requests for additional information, and ultimately receiving a registration certificate to market the device in Russia. The process can take 6-12 months depending on the device class.
Canada medical device approval chart - EMERGO

1. To market medical devices in Canada, manufacturers must classify their device according to regulations, implement an ISO 13485 quality management system with additional Canadian requirements, and obtain ISO 13485 certification from an accredited registrar.
2. Manufacturers then prepare license applications, supporting documents, and pay fees, with process times ranging from 1-8 months depending on device class.
3. Licenses must be renewed annually by meeting regulatory requirements and paying fees to avoid revocation.
India medical device regulatory process

The regulatory framework for medical devices in India is based on drug regulations under the Drugs and Cosmetics Act of 1940 and Drugs and Cosmetics Rules of 1945. The Drug Controller General of India (DCGI) within the Central Drugs Standard Control Organization (CDSCO) regulates medical devices and IVDs. Currently only a limited number of medical device and IVD products require registration in India, including ablation devices, dental implants, and hernia mesh. The registration process for notified devices can take 9-12 months and involves appointing an authorized agent, compiling an application, and obtaining CDSCO approval.
Medical device approval chart for Mexico - Emergo 

In Mexico, medical devices and in-vitro diagnostic devices are regulated by COFEPRIS, a division of the Mexican Ministry of Health. The regulatory process involves classifying the device, appointing a Mexico Registration Holder representative, preparing a registration dossier in Spanish including documentation of quality management and technical specifications, and submitting the application to COFEPRIS for review. Approval time ranges from 1-10 months depending on the device class and review process. Registrations are valid for 5 years and must be renewed in advance of the expiration date.
Europe IVD medical registration and approval chart - EMERGO

The document summarizes the regulatory process for in vitro diagnostic devices (IVDs) in Europe under the In Vitro Diagnostic Directive (98/79/EC). It outlines the classification of IVDs, requirements for quality management systems and technical files, roles of notified bodies and authorized representatives, and timelines and costs associated with the approval process depending on the IVD classification. The process can take from 3-5 months for self-certified IVDs to 9-12 months for list A IVDs and involves implementing quality systems, obtaining notified body audits, and registering with authorities.
US FDA medical device approval chart - Emergo 

Simple one page chart shows the US FDA medical device approval process for Class 1, 2 and 3 devices. Very easy to understand.
Australia medical device registration and approval process - EMERGO

1. To register a medical device in Australia, manufacturers must first determine the device's classification using Australia's regulations and submit an application through an Australian sponsor.
2. The sponsor will submit the application along with supporting documents to the Therapeutic Goods Administration (TGA) for review and approval.
3. If approved, the TGA will provide an Australian Register of Therapeutic Goods (ARTG) listing number allowing marketing in Australia. Listings do not expire as long as annual fees are paid and the device is not changed.
Saudi arabia medical device regulatory process

To market medical devices in Saudi Arabia, devices must receive marketing authorization from the Saudi Food and Drug Authority (SFDA). The SFDA application is reviewed for completeness then sent to a third party for technical review, which can involve multiple rounds of questions. Once approved, the SFDA issues a certificate allowing marketing in Saudi Arabia. Appointing an authorized local representative is also required to manage the registration process. Overall, registration times range from 1-6 months but costs and complexity vary depending on the device's risk classification.
Japan Medical Device Regulatory Approval Process

Before deciding to sell your device in the Japanese market, it is important to understand how the regulations apply to your device, which steps to take, and what resources are required to complete the process. In this presentation, Ann Marie Boullie, Vice President of Business Development for EMERGO, outlines some of the most complex aspects of the Japanese registration process, including:
JMDN codes: device classification and predicates
Clinical data requirements and PMDA pre-submission meetings
Registration routes (Todokede, Ninsho, Shonin)
QMS (Ordinance 169) requirements
Role of the Marketing Authorization Holder (MAH)
Canadaapprovalprocess final13june2012-130116090730-phpapp01

The document discusses the medical device registration process in Canada. It outlines the key steps, which include determining the device classification, obtaining necessary licenses for manufacturers and distributors, implementing quality systems according to ISO 13485, undergoing regulatory audits, and preparing required documentation for premarket review. The process varies depending on the risk class of the device and whether the applicant is a manufacturer, distributor or importer.
Europe CE Marking for medical devices under new MDR

Starting in early 2020, medical devices seeking CE Marking certification in Europe must comply with the new Medical Device Regulation (MDR). This will require appointing personnel responsible for regulatory compliance, classifying the device, appointing an Authorized Representative located in the EU, including labeling with this information, and obtaining a Single Registration Number. Manufacturers must also prepare technical documentation, implement a quality management system, and undergo annual audits by a Notified Body to maintain certification.
Regulation of medical device in japan

Medical Devices registration in Japan , quality system requirements and evaluation and investigation of medical devices in regulated countries ASEAN, China JAPAN and WHO regulations. quality and ethical considerations regulatory and documentation requirements for marketing medical devices and IVDs in regulated countries.
South Korea medical device approval chart - Emergo 

The regulatory process for medical devices in South Korea requires manufacturers to determine the classification of their device and submit the appropriate registration application and supporting documentation to the Ministry of Food and Drug Safety (MFDS). For new devices without a predicate, manufacturers must submit clinical data as part of a Clinical Data Review. Class II devices considered "Special Equivalent" have an expediated review process without a Technical File requirement. Prior international testing may satisfy some requirements but additional Korea-specific testing is often necessary. The MFDS may request additional information or audit submissions, extending approval timelines.
Regulatory requirements for CE CERTIFICATION of Medical Devices in European U...

The EU introduced the CE marking scheme to make trade easier and cheaper between EU countries. It means that a manufacturer claims that their product conforms to the minimum legal requirements for health and safety as laid down in EU directives. In addition, it may be considered a benefit that by implementing the requirements, the product will be safer for the user and this may also reduce damage and liability claims. Additional benefits may include your product being made safer for end-users. The EU introduced the CE marking scheme to make trade easier and cheaper between EU countries. It means that a manufacturer claims that their product conforms to the minimum legal requirements for health and safety as laid down in EU directives. If you manufacture or import a product which falls within the scope of one or more of the New Approach Directives and wish to place your product on the market in any of the member’s states of the European Economic Area (EEA), then you must apply CE marking to your product against the essential requirements of all these applicable directives.
Key Words: European Union, CE marking, New Approach Directives, EEA, Regulatory requirements.
Medical devices

To comprehend the regulatory requirements to import medical Medical devices and authorization procedures in regulated markets of the United States and Australia
Regulatory approval process for invitro diagnostics in us

This document summarizes the regulatory approval process for in vitro diagnostics (IVDs) in the United States. IVDs are classified into Class I, II, or III based on risk, with Class III devices requiring premarket approval. The main regulatory pathways for approval are 510(k) premarket notification for demonstrating substantial equivalence to a predicate device or the premarket approval (PMA) process for novel high-risk devices. Clinical Laboratory Improvement Amendments also provide quality standards for lab testing. The document reviews the classification system and options for 510(k), PMA, de novo, and lab developed tests.
The European Medical Device Regulations - analysis of the final text

Following the publication of the new European Medical Device Regulations by the European Commission, we offer you our analysis of the final text.
Medical device regulations in india

A brief introduction on medical device , how iit is regulated in india,its marketing authorization,classification, notified devices,import of device and registration process, fnctions of medical device department.
China\'s Medical Device Regulatory Process

This presentation is an overview of the Medical Device Regulatory Process for China. I used this to teach about SFDA Order No. 27, the amendments, Order No. 15 (Classification), Order No. 16 (Registration), YY, GB, YY/T, GB/T, YZB standards and the different registration forms to use for devices made in People\'s Republic of China, about imported medical devices, and about devices allowed from Taiwan, Hong Kong and Macau regions.
CE marking and CE certification

CE marking and CE certification what is it why you need it who can apply
CE marking certification for medical devices. Medical Device Regulations. It Is Easy To Make Mistakes In The Regulatory Process That Can Delay.
Visit: http://www.meddevicecorp.com/
510kvs pma slides

The document discusses the FDA's regulatory pathways for medical devices. The FDA uses a risk-based classification system to categorize devices as Class I, II, or III based on risk, with Class III devices posing the highest risk. Class I devices face the fewest regulatory requirements while Class III devices require a rigorous premarket approval process. The key pathways are 510(k) clearance for Class II devices and premarket approval (PMA) for Class III devices. The FDA aims to evaluate devices throughout their lifecycle from premarket through postmarket surveillance to ensure safety and effectiveness.
Regulatory Approval Process for Medical Devices in EU - Presentation by Aksha...

A presentation on Regulatory Approval Process for Medical Devices in European Union that explains in brief about the various aspects including the EU Medical Device Directives, Classifications, CE Certification, Medical Device Registration & Timelines. This was presented as a part of curriculum by Akshay Anand in JSS College of Pharmacy, Mysuru during January 2015
Medical devices CHINA 

Introduction
Definition
Classification
Regulatory registration procedure
Quality system requirements
Clinical evaluation
Investigation of medical devices
Summary
Medical devices are regulated by the National Medical Product Administration (NMPA), formerly the China Food and Drug Administration (CFDA)
Manufacturers must register their devices with the NMPA before selling or distributing in China.
The NMPA reviews all device applications and has strict requirements for submission documentation, testing, and clinical data.
Any instrument, apparatus, appliance, material, or other article whether used alone or in combination, including the software necessary for its proper application.
Diagnosis, prevention, monitoring, treatment or alleviation of disease
Diagnosis, monitoring, treatment, alleviation of compensation for an injury or handicap conditions
Investigation, replacement or modification for anatomy or a physiological process
Control of conception
The Chinese authorities (CFDA/NMPA) have their own quality management system requirements.
However, these “GMP requirements” are very similar to ISO 13485.
Therefore, manufacturers usually submit the ISO 13485 certificate.
However, the audit will review this certificate against the Chinese GMP requirements.
These audits are regularly carried out during the approval procedure and/or after a recall.
NMPA issued and implemented the "Guideline on Inspection of Quality Management System for Medical Device Registration" on October 10, 2022.
Product Life-cycle Process
The guideline specifies the basic requirements for registration inspection, self-inspection, commissioned inspection, and extended inspection, etc. Applicant needs to:
Ensure the design, development, production, and other process data to be true, accurate, complete, and traceable, and consistent with the registration application materials.
Carry out the registration QMS inspection in reference to the registration application materials, and focus on the design, development, procurement, production management, and quality control of the product.
For lower-risk medical devices (Class I and Class II), clinical evaluation may not always be required. However, higher-risk devices (Class III and implantable devices) generally require clinical evaluation.
The evaluation involves reviewing existing clinical data, scientific literature, and relevant clinical research to assess the safety and performance of the device.
All clinical trials for medical devices must follow China Good Clinical Practices
Clinical investigations are required if no equivalent devices can be found and safety and efficacy cannot be proven with other clinical and non-clinical data.
For certain medical devices, the NMPA may require clinical investigations, especially for novel devices or those with significant risk.
Clinical investigations involve conducting studies on human subjects to generate clinical data on the safety and effectiveness of the device.
Colombia medical device approval chart - Emergo 

The Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA) governs medical devices in Colombia. To register a device, a manufacturer must determine its classification, provide documentation such as quality and safety certificates, and submit an application through an Importer or Legal Representative. Approval times range from automatic for Class I and IIa devices to 4-6 months for Class IIb and III. Once approved, registrations are valid for 10 years.
Japan PMDA Medical Device Regulatory Approval Process

Watch the recorded webinar: https://www.emergogroup.com/resources/video-webinar-japan-registration-process
Japan's medical device market is one of the most robust markets in Asia, but its regulatory system can be confusing. Before deciding to sell your device in the Japanese market, it is important to understand how the regulations apply to your device, which steps to take, and what resources are required to complete the process. In this presentation, Ann Marie Boullie, Vice President of Business Development for EMERGO, will discuss some of the most complex aspects of the Japanese registration process, including:
JMDN codes: device classification and predicates
Clinical data requirements and PMDA pre-submission meetings
Registration routes (Todokede, Ninsho, Shonin)
QMS (Ordinance 169) requirements
Role of the Marketing Authorization Holder (MAH)
Plus more ...
Digital & Social Marketing for Medical Devices

The Internet has revolutionized the way the consumer market functions. How is it impacting the way in which medical devices are developed, funded and marketed? Speak with industry experts and learn how you can develop your company’s Internet strategy.
Medical devices IP and marketing invitation

An invitation to our special event on Medical Devices - the secrets behind strong IP & Smart Marketing
More Related Content
What's hot
US FDA medical device approval chart - Emergo 

Simple one page chart shows the US FDA medical device approval process for Class 1, 2 and 3 devices. Very easy to understand.
Australia medical device registration and approval process - EMERGO

1. To register a medical device in Australia, manufacturers must first determine the device's classification using Australia's regulations and submit an application through an Australian sponsor.
2. The sponsor will submit the application along with supporting documents to the Therapeutic Goods Administration (TGA) for review and approval.
3. If approved, the TGA will provide an Australian Register of Therapeutic Goods (ARTG) listing number allowing marketing in Australia. Listings do not expire as long as annual fees are paid and the device is not changed.
Saudi arabia medical device regulatory process

To market medical devices in Saudi Arabia, devices must receive marketing authorization from the Saudi Food and Drug Authority (SFDA). The SFDA application is reviewed for completeness then sent to a third party for technical review, which can involve multiple rounds of questions. Once approved, the SFDA issues a certificate allowing marketing in Saudi Arabia. Appointing an authorized local representative is also required to manage the registration process. Overall, registration times range from 1-6 months but costs and complexity vary depending on the device's risk classification.
Japan Medical Device Regulatory Approval Process

Before deciding to sell your device in the Japanese market, it is important to understand how the regulations apply to your device, which steps to take, and what resources are required to complete the process. In this presentation, Ann Marie Boullie, Vice President of Business Development for EMERGO, outlines some of the most complex aspects of the Japanese registration process, including:
JMDN codes: device classification and predicates
Clinical data requirements and PMDA pre-submission meetings
Registration routes (Todokede, Ninsho, Shonin)
QMS (Ordinance 169) requirements
Role of the Marketing Authorization Holder (MAH)
Canadaapprovalprocess final13june2012-130116090730-phpapp01

The document discusses the medical device registration process in Canada. It outlines the key steps, which include determining the device classification, obtaining necessary licenses for manufacturers and distributors, implementing quality systems according to ISO 13485, undergoing regulatory audits, and preparing required documentation for premarket review. The process varies depending on the risk class of the device and whether the applicant is a manufacturer, distributor or importer.
Europe CE Marking for medical devices under new MDR

Starting in early 2020, medical devices seeking CE Marking certification in Europe must comply with the new Medical Device Regulation (MDR). This will require appointing personnel responsible for regulatory compliance, classifying the device, appointing an Authorized Representative located in the EU, including labeling with this information, and obtaining a Single Registration Number. Manufacturers must also prepare technical documentation, implement a quality management system, and undergo annual audits by a Notified Body to maintain certification.
Regulation of medical device in japan

Medical Devices registration in Japan , quality system requirements and evaluation and investigation of medical devices in regulated countries ASEAN, China JAPAN and WHO regulations. quality and ethical considerations regulatory and documentation requirements for marketing medical devices and IVDs in regulated countries.
South Korea medical device approval chart - Emergo 

The regulatory process for medical devices in South Korea requires manufacturers to determine the classification of their device and submit the appropriate registration application and supporting documentation to the Ministry of Food and Drug Safety (MFDS). For new devices without a predicate, manufacturers must submit clinical data as part of a Clinical Data Review. Class II devices considered "Special Equivalent" have an expediated review process without a Technical File requirement. Prior international testing may satisfy some requirements but additional Korea-specific testing is often necessary. The MFDS may request additional information or audit submissions, extending approval timelines.
Regulatory requirements for CE CERTIFICATION of Medical Devices in European U...

The EU introduced the CE marking scheme to make trade easier and cheaper between EU countries. It means that a manufacturer claims that their product conforms to the minimum legal requirements for health and safety as laid down in EU directives. In addition, it may be considered a benefit that by implementing the requirements, the product will be safer for the user and this may also reduce damage and liability claims. Additional benefits may include your product being made safer for end-users. The EU introduced the CE marking scheme to make trade easier and cheaper between EU countries. It means that a manufacturer claims that their product conforms to the minimum legal requirements for health and safety as laid down in EU directives. If you manufacture or import a product which falls within the scope of one or more of the New Approach Directives and wish to place your product on the market in any of the member’s states of the European Economic Area (EEA), then you must apply CE marking to your product against the essential requirements of all these applicable directives.
Key Words: European Union, CE marking, New Approach Directives, EEA, Regulatory requirements.
Medical devices

To comprehend the regulatory requirements to import medical Medical devices and authorization procedures in regulated markets of the United States and Australia
Regulatory approval process for invitro diagnostics in us

This document summarizes the regulatory approval process for in vitro diagnostics (IVDs) in the United States. IVDs are classified into Class I, II, or III based on risk, with Class III devices requiring premarket approval. The main regulatory pathways for approval are 510(k) premarket notification for demonstrating substantial equivalence to a predicate device or the premarket approval (PMA) process for novel high-risk devices. Clinical Laboratory Improvement Amendments also provide quality standards for lab testing. The document reviews the classification system and options for 510(k), PMA, de novo, and lab developed tests.
The European Medical Device Regulations - analysis of the final text

Following the publication of the new European Medical Device Regulations by the European Commission, we offer you our analysis of the final text.
Medical device regulations in india

A brief introduction on medical device , how iit is regulated in india,its marketing authorization,classification, notified devices,import of device and registration process, fnctions of medical device department.
China\'s Medical Device Regulatory Process

This presentation is an overview of the Medical Device Regulatory Process for China. I used this to teach about SFDA Order No. 27, the amendments, Order No. 15 (Classification), Order No. 16 (Registration), YY, GB, YY/T, GB/T, YZB standards and the different registration forms to use for devices made in People\'s Republic of China, about imported medical devices, and about devices allowed from Taiwan, Hong Kong and Macau regions.
CE marking and CE certification

CE marking and CE certification what is it why you need it who can apply
CE marking certification for medical devices. Medical Device Regulations. It Is Easy To Make Mistakes In The Regulatory Process That Can Delay.
Visit: http://www.meddevicecorp.com/
510kvs pma slides

The document discusses the FDA's regulatory pathways for medical devices. The FDA uses a risk-based classification system to categorize devices as Class I, II, or III based on risk, with Class III devices posing the highest risk. Class I devices face the fewest regulatory requirements while Class III devices require a rigorous premarket approval process. The key pathways are 510(k) clearance for Class II devices and premarket approval (PMA) for Class III devices. The FDA aims to evaluate devices throughout their lifecycle from premarket through postmarket surveillance to ensure safety and effectiveness.
Regulatory Approval Process for Medical Devices in EU - Presentation by Aksha...

A presentation on Regulatory Approval Process for Medical Devices in European Union that explains in brief about the various aspects including the EU Medical Device Directives, Classifications, CE Certification, Medical Device Registration & Timelines. This was presented as a part of curriculum by Akshay Anand in JSS College of Pharmacy, Mysuru during January 2015
Medical devices CHINA 

Introduction
Definition
Classification
Regulatory registration procedure
Quality system requirements
Clinical evaluation
Investigation of medical devices
Summary
Medical devices are regulated by the National Medical Product Administration (NMPA), formerly the China Food and Drug Administration (CFDA)
Manufacturers must register their devices with the NMPA before selling or distributing in China.
The NMPA reviews all device applications and has strict requirements for submission documentation, testing, and clinical data.
Any instrument, apparatus, appliance, material, or other article whether used alone or in combination, including the software necessary for its proper application.
Diagnosis, prevention, monitoring, treatment or alleviation of disease
Diagnosis, monitoring, treatment, alleviation of compensation for an injury or handicap conditions
Investigation, replacement or modification for anatomy or a physiological process
Control of conception
The Chinese authorities (CFDA/NMPA) have their own quality management system requirements.
However, these “GMP requirements” are very similar to ISO 13485.
Therefore, manufacturers usually submit the ISO 13485 certificate.
However, the audit will review this certificate against the Chinese GMP requirements.
These audits are regularly carried out during the approval procedure and/or after a recall.
NMPA issued and implemented the "Guideline on Inspection of Quality Management System for Medical Device Registration" on October 10, 2022.
Product Life-cycle Process
The guideline specifies the basic requirements for registration inspection, self-inspection, commissioned inspection, and extended inspection, etc. Applicant needs to:
Ensure the design, development, production, and other process data to be true, accurate, complete, and traceable, and consistent with the registration application materials.
Carry out the registration QMS inspection in reference to the registration application materials, and focus on the design, development, procurement, production management, and quality control of the product.
For lower-risk medical devices (Class I and Class II), clinical evaluation may not always be required. However, higher-risk devices (Class III and implantable devices) generally require clinical evaluation.
The evaluation involves reviewing existing clinical data, scientific literature, and relevant clinical research to assess the safety and performance of the device.
All clinical trials for medical devices must follow China Good Clinical Practices
Clinical investigations are required if no equivalent devices can be found and safety and efficacy cannot be proven with other clinical and non-clinical data.
For certain medical devices, the NMPA may require clinical investigations, especially for novel devices or those with significant risk.
Clinical investigations involve conducting studies on human subjects to generate clinical data on the safety and effectiveness of the device.
Colombia medical device approval chart - Emergo 

The Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA) governs medical devices in Colombia. To register a device, a manufacturer must determine its classification, provide documentation such as quality and safety certificates, and submit an application through an Importer or Legal Representative. Approval times range from automatic for Class I and IIa devices to 4-6 months for Class IIb and III. Once approved, registrations are valid for 10 years.
Japan PMDA Medical Device Regulatory Approval Process

Watch the recorded webinar: https://www.emergogroup.com/resources/video-webinar-japan-registration-process
Japan's medical device market is one of the most robust markets in Asia, but its regulatory system can be confusing. Before deciding to sell your device in the Japanese market, it is important to understand how the regulations apply to your device, which steps to take, and what resources are required to complete the process. In this presentation, Ann Marie Boullie, Vice President of Business Development for EMERGO, will discuss some of the most complex aspects of the Japanese registration process, including:
JMDN codes: device classification and predicates
Clinical data requirements and PMDA pre-submission meetings
Registration routes (Todokede, Ninsho, Shonin)
QMS (Ordinance 169) requirements
Role of the Marketing Authorization Holder (MAH)
Plus more ...
What's hot (20)
Australia medical device registration and approval process - EMERGO

Australia medical device registration and approval process - EMERGO
Canadaapprovalprocess final13june2012-130116090730-phpapp01

Canadaapprovalprocess final13june2012-130116090730-phpapp01
Europe CE Marking for medical devices under new MDR

Europe CE Marking for medical devices under new MDR
South Korea medical device approval chart - Emergo 

South Korea medical device approval chart - Emergo
Regulatory requirements for CE CERTIFICATION of Medical Devices in European U...

Regulatory requirements for CE CERTIFICATION of Medical Devices in European U...
Regulatory approval process for invitro diagnostics in us

Regulatory approval process for invitro diagnostics in us
The European Medical Device Regulations - analysis of the final text

The European Medical Device Regulations - analysis of the final text
Regulatory Approval Process for Medical Devices in EU - Presentation by Aksha...

Regulatory Approval Process for Medical Devices in EU - Presentation by Aksha...
Japan PMDA Medical Device Regulatory Approval Process

Japan PMDA Medical Device Regulatory Approval Process
Viewers also liked
Digital & Social Marketing for Medical Devices

The Internet has revolutionized the way the consumer market functions. How is it impacting the way in which medical devices are developed, funded and marketed? Speak with industry experts and learn how you can develop your company’s Internet strategy.
Medical devices IP and marketing invitation

An invitation to our special event on Medical Devices - the secrets behind strong IP & Smart Marketing
6 Steps to Global Markets

As you start to map out your global strategy beyond the US, Canada and Europe, there are six key steps to follow and multiple regulations to consider. Harmonization has increased in recent years, yet most countries still maintain distinct medical device regulatory systems. Careful analysis on a market-by-market basis will help you to fully realize compliance requirements affecting a particular market. Explore these six steps as we reveal insights into the unique medical device regulations affecting markets in Russia, Brazil, Japan, China, and India.
Choosing the right facility for clinical trials in Russia

This document provides information on conducting clinical trials in Russia. It notes that medical institutions must be accredited by Roszdravnadzor to perform clinical trials. Main medical institutions in central Russia are preferable as they tend to have wider specializations and can perform trials faster. Roszdravnadzor oversees the accreditation process, maintains a list of accredited facilities, and verifies that facilities have the proper licenses and equipment before issuing permission to conduct trials. Facilities must submit documentation to Roszdravnadzor to become accredited and receive approval to perform specific clinical trials.
Clinical Trials in Russia

Clinical trials are required in Russia to legally sell a medical device. The document outlines the regulatory basis and process for conducting clinical trials in Russia. There are two types of clinical trials - ones conducted on patients and ones involving analysis of literature sources. Clinical trials involve developing a plan, getting ethics committee approval, and permission from regulatory authorities. The applicant must provide documentation on the device and its testing to the medical institution conducting the clinical trials. A program and duration of clinical trials is then coordinated based on the device's purpose and complexity.
Let us put some clarity on VAT topic

This document outlines the value-added tax (VAT) rates for medical devices in Russia both past and present. It states that historically, medical equipment was subject to a 0% VAT rate, medical products had an 18% VAT rate, and the exact lists of qualifying items were defined in government orders from 2002 and 2008. As of January 1, 2013, a new law unified these terms as "medical devices" and introduced some VAT rate changes, making the VAT rules less clear. The document concludes that additional low-level legislative changes on medical device VAT rates in Russia may still be forthcoming.
Localization 2014

The Russian Ministry of Industry and Trade reconsidered restrictions on the supply of medical devices for state procurement in July 2014. The restrictions affect state and municipal procurements of medical devices from a closed list. If at least two bids with local products from Russia, Belarus or Kazakhstan are submitted for tender, buyers will be unable to procure medical devices originating from other countries. The local products must be confirmed by a certificate of origin issued by a local authorized agency. Certain categories of medical devices are subject to the restrictions, including syringes, osteosynthesis implants, ECG devices, and surgical instruments.
Changes and Modifications

This document summarizes regulations for changes and modifications to registered medical devices across Russia, Kazakhstan, and Belarus. It outlines three procedures - amendment, notification, and re-registration. An amendment procedure allows for name, address, or accessory changes and is fastest in Russia and Kazakhstan but requires full re-registration in Belarus. A notification procedure in Russia addresses certain indication, storage, marking, or software changes and is approved within 30 days. All other changes generally require the full re-registration process.
Viewers also liked (8)
Digital & Social Marketing for Medical Devices

Digital & Social Marketing for Medical Devices
Choosing the right facility for clinical trials in Russia

Choosing the right facility for clinical trials in Russia
Similar to Japan medical device approval chart - Emergo
Pakistan Medical Device regulatory process

Medical devices in Pakistan are regulated by the Medical Device & Medicated Cosmetics Division and must be classified according to the Medical Device Rules of 2017. The regulatory process involves appointing a registered importer, determining the classification, and having an authorized representative submit an application dossier and fees to the Medical Devices Board for approval. Approval times range from less than two months for Class A local manufacturer devices to 8-10 months for Class D imported devices not from reference countries.
Key Differences between Medical Device User Fees and Establishment Registrati...

This document compares medical device user fees and establishment registration and listing requirements with the FDA. Medical device user fees are paid by applicants submitting applications for product review, such as 510(k)s or PMAs. Establishment registration fees are paid annually by companies involved in medical device production and distribution. Key differences include what entities must pay each fee, annual versus multi-year fee structures, exemptions for small businesses from some user fees, and different processes for payment and submission of registration information. Understanding the distinction between these fee types is important for medical device companies to comply with FDA regulatory requirements.
Registration of medical devices with Brazil's Anvisa 

overview of Anvisa Requirements, Medical device classification and grouping, cadostro registration pathway,resgistro pathway,INMETRO Certification, Summary of Anvisa Registration Process
Overview of Japanese Foreign Manufacturers Accreditation

This document discusses the process for foreign manufacturers to obtain accreditation from the Japanese Ministry of Health, Labour and Welfare to export medical devices to Japan. It outlines the requirements, application process, and fees for initial accreditation, changes to an existing accreditation, renewal of accreditation, and notifications of changes. The accreditation process involves submitting application documents to both the Ministry and the Pharmaceutical and Medical Devices Agency, who reviews facilities and processes to ensure compliance with Japanese regulations.
Medical Device Administration In China

This document discusses medical device administration in China. It outlines the competent authorities that regulate medical devices, including the SFDA and Center for Medical Device Evaluation. It also describes the administration activities like registration, production, sales, and post-marketing surveillance. The document then summarizes the laws and regulations regarding medical devices and classifies medical devices into three categories based on risk. It provides details on administration of production, sales, and registration of medical devices in China.
Medical Device Regulatory in Asia_Brazil

For more information:
Email: info@arqon.com
LinkedIn : ARQon - Asia Regulatory and Quality Consultancy for Medical Device and Drugs
Twitter: @ARQonSG
Medical device regulatory & registration in Asia/ASEAN: Australia, Cambodia, China, Hong Kong, India, Indonesia, Japan, Malaysia, Myanmar, Nepal, New Zealand, Philippines, Singapore, South Korea, Taiwan, Thailand, Vietnam
-ARQon Service-
ARQon (Asia Regulatory & Quality Consultancy) is a regulatory consultancy firm for medical devices and drug companies. We assist our clients in areas of regulatory and quality from product development to product registration to attain market approval in ASIA, ASEAN, EU, US, and the Rest of the World.
ASIA - Austria TGA, Taiwan FDA, New Zealand Medsafe, Japan MHLW, South Korea MFDS, Hong Kong MDCO, Pakistan DRA, Bangladesh DGDA, China FDA, India CDSCO Approvals
ASEAN - Singapore HSA, Cambodia DDF, MOH Indonesia, Malaysia MDA, Myanmar FDA, Philippines FDA, Thai FDA, Vietnam DMEC Approvals
AMERICAS - Brazil ANVISA, Health Canada, Colombia INVIMA, Costa Rica MOH, Mexico COFEPRIS, Peru DIGEMID, US FDA Approvals
EUROPE - Notified Bodies Approval
MIDDLE EAST - Egypt CAPA, Emirates MOH, Saudi Arabia FDA Approvals
How to start a medical device business in india

Nowadays, health is given much priority in all aspects. The present
generation is very much conscious about having periodic checkups
and maintaining a good health. This awareness has given an upper
hand to the medical equipment business. The healthcare industry is
booming like no other field and because of this, Medical Device
Regulation India has also become equally complicated. Unlike
previous years, nowadays most medical devices are required to be
registered and need approval for business
New drug approval

The document discusses India's drug regulatory system. The Drug Controller General of India regulates drugs and medical devices in the country to ensure quality, safety and efficacy. New drugs require approval through a New Drug Application process which involves submitting documentation on manufacturing, non-clinical studies, and clinical trials for review. It takes about a year to review an NDA and various forms and fees are involved in the approval and import license application processes.
Good Regulatory Practice.pptx

Good regulatory practice are internationally recognized process, system, tools and method for improving the quality of regulation.
It includes
1)COMMUNITY PHARMACY RETAIL AND WHOLESALE PHARMACY
-Dacumentation
-Licenses
-Renewal
-E-governance
2) HOSPITAL PHARMACY
-Dacumentation
-Licenses
-Renewal
-E-governance
3) PHARMA MANIFACTURING
-Dacumentation
-Licenses
-Renewal
-E-governance
4) IMPORT OF DRUG AND MEDICAL DEVICE
-Dacumentation
-Licenses
-Renewal
-E-governance
5) EXPORT OF DRUG AND MEDICAL DEVICE
-Dacumentation
-Licenses
-Renewal
-E-governance
JAPANESE MARKET OF MEDICAL DEVICES ANUP

The Japanese market for medical devices reached $37.6 billion in 2016 and is expected to grow to $45 billion by 2020, making it the second largest market globally in terms of growth rate. It remains heavily reliant on imports, especially for sophisticated devices. The regulatory agencies overseeing the medical device market in Japan are the Pharmaceuticals and Medical Devices Agency (PMDA) and the Ministry of Health, Labor and Welfare (MHLW). Devices are classified based on risk into four classes, with Class I having the lowest risk and Class IV the highest. Regulatory approval processes differ depending on the class, ranging from self-declaration for Class I devices to pre-market approval from MHLW for Class III and
REGULATORY REQUIREMENTS FOR REGISTRATION OF DRUGS AND POST APPROVAL REQUIREME...

The document discusses regulatory requirements for drug registration and post-approval requirements in Saudi Arabia. It provides information on the Gulf Cooperation Council agreement between six Middle Eastern countries to harmonize regulatory standards. The Saudi Food and Drug Authority regulates drugs in Saudi Arabia, requiring electronic submissions through its eCTD format. Drugs must undergo assessment of quality, safety and efficacy to receive approval and be licensed for marketing. Post-approval, variations and renewals are regulated, and fees apply to the different application and registration types.
5-MHRA(2).pdf

The MHRA has sent a letter to Peter Wei of Lotus Global Co Ltd confirming registration of Xiamen Jiqing Biomedical Technology Co., Ltd. as a manufacturer of in vitro diagnostic medical devices based on the information provided by Wei as their authorized representative. The letter notes that registration does not represent endorsement of the manufacturer's device classifications and that any changes to the registration information must be reported.
Strategies for Device Approval in China, India, South Korea and Australia

This session will describe the orthopaedic device regulatory and registration requirements in Asia Pacific markets. Regulatory steps and strategies will be presented for each of these countries. The discussion will also cover ways to gain regulatory information about competitors already selling in these markets. Attendees will leave the session with an understanding of timelines, costs and complexity for approval.
Copp- Certificate of Pharmaceutical Products

The document provides guidelines for applying for a WHO GMP Certificate of Pharmaceutical Product (COPP) from the Indian drug regulatory authority, CDSCO. An application for a COPP must be submitted to the respective CDSCO zonal/sub-zonal office, along with documents like a product permission copy, notarized product summary sheet, and documentation of quality control procedures. CDSCO officers will inspect applicant sites to ensure compliance with WHO GMP guidelines before issuing a COPP, which certifies that a pharmaceutical product is manufactured according to proper standards for international distribution and registration.
Exam no. 76 ashma

This document summarizes the marketing authorization procedures and post-approval changes (variations) for medicinal products in the UK. There are three procedures for obtaining initial marketing authorization: the decentralized procedure, mutual recognition procedure, and centralized procedure. After approval, post-approval changes or variations can be made, categorized as either type I (minor), type II (major), or type III (line extension). Supporting documents must be submitted to the appropriate authority for review and approval of variations before they are implemented.
CE Marking , FDA Approval and Associated Regulations for Wellness

This document discusses key regulatory considerations for medical device approval in major markets. It provides an overview of regulatory strategies and approval processes in the US, EU, Canada and other countries. Major topics covered include device classification systems, premarket approval requirements, quality management standards, clinical data requirements and post-market obligations. Common mistakes made by companies and the importance of developing a comprehensive regulatory strategy early on are also addressed.
Regulatory mechanism in india

The Central Drugs Standard Control Organization (CDSCO) is the key medical regulatory organization in India and has established a Medical Device Division to oversee matters related to notified medical devices. The Central Licensing Approval Authority (CLAA) serves as the main regulatory body for medical devices. All medical devices must undergo conformity assessment to ensure safety and quality standards are met. Obtaining registration for a medical device in India requires appointing a local representative, compiling a device application, undergoing CDSCO review which may include queries and a technical presentation, and can take 6-9 months or up to 18 months if clinical trials are required. Registration is valid for 3 years and must be renewed in advance of expiry. An import license from the CDSCO
Importing and exporting medical devices

This document provides an overview of the requirements for importing and exporting medical devices. Any medical device legally in the US can be exported without FDA approval, but devices not approved in the US must follow export provisions. Foreign manufacturers importing devices into the US must comply with FDA regulations. Imported devices must also meet Customs requirements and may be detained if noncompliant. The webinar will cover import/export requirements, regulations, entry documentation, and exporting approved and unapproved devices. It is intended to benefit those in regulatory, clinical, quality, marketing, and other roles involved in imports and exports.
COPP.pptx

The document provides information about Certificates of Pharmaceutical Product (COPP):
- A COPP is issued by national health authorities in the exporting country upon request from authorities or suppliers in the importing country. It certifies that a pharmaceutical product complies with Good Manufacturing Practices and is approved for sale in the exporting country.
- The COPP allows registration and marketing of the product in the importing country and is necessary for registration renewal. It helps authorities in importing countries assess product quality for import/registration.
- The application process involves submitting documents like manufacturing licenses and quality manuals. Facilities undergo inspection to verify compliance with WHO GMP guidelines. Certifications are issued for approved products and may expire after 24
medical device registration in china

The document provides an overview of medical device registration in China. It discusses the Chinese regulatory environment and key organizations like the China Food and Drug Administration (CFDA). The CFDA classifies medical devices and oversees the registration process, which involves choosing an agent, submitting documentation, conducting tests, making two submissions for review, and final administrative approval. Attention to small details and unpredictability are characteristics of the Chinese process.
Similar to Japan medical device approval chart - Emergo (20)
Key Differences between Medical Device User Fees and Establishment Registrati...

Key Differences between Medical Device User Fees and Establishment Registrati...
Registration of medical devices with Brazil's Anvisa 

Registration of medical devices with Brazil's Anvisa
Overview of Japanese Foreign Manufacturers Accreditation

Overview of Japanese Foreign Manufacturers Accreditation
REGULATORY REQUIREMENTS FOR REGISTRATION OF DRUGS AND POST APPROVAL REQUIREME...

REGULATORY REQUIREMENTS FOR REGISTRATION OF DRUGS AND POST APPROVAL REQUIREME...
Strategies for Device Approval in China, India, South Korea and Australia

Strategies for Device Approval in China, India, South Korea and Australia
CE Marking , FDA Approval and Associated Regulations for Wellness

CE Marking , FDA Approval and Associated Regulations for Wellness
Recently uploaded
Innovative Minds France's Most Impactful Healthcare Leaders.pdf

This edition features a handful of Innovative Minds: France's Most Impactful Healthcare Leaders that are leading us into a better future.
Can Allopathy and Homeopathy Be Used Together in India.pdf

This article explores the potential for combining allopathy and homeopathy in India, examining the benefits, challenges, and the emerging field of integrative medicine.
Tips for Pet Care in winters How to take care of pets.

Tips for Pet Care in winters How to take care of pets.
PET CT beginners Guide covers some of the underrepresented topics in PET CT

This lecture briefly covers some of the underrepresented topics in Molecular imaging with cases , such as:
- Primary pleural tumors and pleural metastases.
- Distinguishing between MPM and Talc Pleurodesis.
- Urological tumors.
- The role of FDG PET in NET.
Letter to MREC - application to conduct study

Application to conduct study on research title 'Awareness and knowledge of oral cancer and precancer among dental outpatient in Klinik Pergigian Merlimau, Melaka'
DRAFT Ventilator Rapid Reference version 2.4.pdf

Ventilator rapid reference for the Zoll Z cvent. Presented for educational purposes only.
The Power of Superfoods and Exercise.pdf

Healthy Eating Habits:
Understanding Nutrition Labels: Teaches how to read and interpret food labels, focusing on serving sizes, calorie intake, and nutrients to limit or include.
Tips for Healthy Eating: Offers practical advice such as incorporating a variety of foods, practicing moderation, staying hydrated, and eating mindfully.
Benefits of Regular Exercise:
Physical Benefits: Discusses how exercise aids in weight management, muscle and bone health, cardiovascular health, and flexibility.
Mental Benefits: Explains the psychological advantages, including stress reduction, improved mood, and better sleep.
Tips for Staying Active:
Encourages consistency, variety in exercises, setting realistic goals, and finding enjoyable activities to maintain motivation.
Maintaining a Balanced Lifestyle:
Integrating Nutrition and Exercise: Suggests meal planning and incorporating physical activity into daily routines.
Monitoring Progress: Recommends tracking food intake and exercise, regular health check-ups, and provides tips for achieving balance, such as getting sufficient sleep, managing stress, and staying socially active.
Professional Secrecy: Forensic Medicine Lecture

Professional Secrecy: Forensic Medicine Lecture , Medical jurisprudence
Michigan HealthTech Market Map 2024 with Policy Makers, Academic Innovation C...

Michigan HealthTech Market Map 2024. Includes 7 categories: Policy Makers, Academic Innovation Centers, Digital Health Providers, Healthcare Providers, Payers / Insurance, Device Companies, Life Science Companies, Innovation Accelerators. Developed by the Michigan-Israel Business Accelerator
Luxurious Spa In Ajman Chandrima Massage Center

Chandrima Spa Ajman is one of the leading Massage Center in Ajman, which is open 24 hours exclusively for men. Being one of the most affordable Spa in Ajman, we offer Body to Body massage, Kerala Massage, Malayali Massage, Indian Massage, Pakistani Massage Russian massage, Thai massage, Swedish massage, Hot Stone Massage, Deep Tissue Massage, and many more. Indulge in the ultimate massage experience and book your appointment today. We are confident that you will leave our Massage spa feeling refreshed, rejuvenated, and ready to take on the world.
Visit : https://massagespaajman.com/
Call : 052 987 1315
PrudentRx's Function in the Management of Chronic Illnesses

PrudentRx ensures effective, cost-efficient medication management for chronic diseases, enhancing patient health and productivity.
Stem Cell Solutions: Dr. David Greene's Path to Non-Surgical Cardiac Care

Explore the groundbreaking work of Dr. David Greene, a pioneer in regenerative medicine, who is revolutionizing the field of cardiology through stem cell therapy in Arizona. This ppt delves into how Dr. Greene's innovative approach is providing non-surgical, effective treatments for heart disease, using the body's own cells to repair heart damage and improve patient outcomes. Learn about the science behind stem cell therapy, its benefits over traditional cardiac surgeries, and the promising future it holds for modern medicine. Join us as we uncover how Dr. Greene's commitment to stem cell research and therapy is setting new standards in healthcare and offering new hope to cardiac patients.
Deep Leg Vein Thrombosis (DVT): Meaning, Causes, Symptoms, Treatment, and Mor...

Deep Leg Vein Thrombosis (DVT): Meaning, Causes, Symptoms, Treatment, and Mor...The Lifesciences Magazine
Deep Leg Vein Thrombosis occurs when a blood clot forms in one or more of the deep veins in the legs. These clots can impede blood flow, leading to severe complications.KEY Points of Leicester travel clinic In London doc.docx

In order to protect visitors' safety and wellbeing, Travel Clinic Leicester offers a wide range of travel-related health treatments, including individualized counseling and vaccines. Our team of medical experts specializes in getting people ready for international travel, with a particular emphasis on vaccines and health consultations to prevent travel-related illnesses. We provide a range of travel-related services, such as health concerns unique to a trip, prevention of malaria, and travel-related medical supplies. Our clinic is dedicated to providing top-notch care, keeping abreast of the most recent recommendations for vaccinations and travel health precautions. The goal of Travel Clinic Leicester is to keep you safe and well-rested no matter what kind of travel you choose—business, pleasure, or adventure.
PrudentRx: A Resource for Patient Education and Engagement

PrudentRx enhances healthcare by educating patients and ensuring effective medication management in a complex, evolving landscape.
Feeding plate for a newborn with Cleft Palate.pptx

A feeding plate is a prosthetic device used for newborns with a cleft palate to assist in feeding and improve nutrition intake. From a prosthodontic perspective, this plate acts as a barrier between the oral and nasal cavities, facilitating effective sucking and swallowing by providing a more normal anatomical structure. It helps to prevent milk from entering the nasal passage, thereby reducing the risk of aspiration and enhancing the infant's ability to feed efficiently. The feeding plate also aids in the development of the oral muscles and can contribute to better growth and weight gain. Its custom fabrication and proper fitting by a prosthodontist are crucial for ensuring comfort and functionality, as well as for minimizing potential complications. Early intervention with a feeding plate can significantly improve the quality of life for both the infant and the parents.
TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardso...

TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardso...rightmanforbloodline
TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardson, Verified Chapters 1 - 18, Complete Newest Version
TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardson, Verified Chapters 1 - 18, Complete Newest Version
TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardson, Verified Chapters 1 - 18, Complete Newest VersionLGBTQ+ Adults: Unique Opportunities and Inclusive Approaches to Care

This webinar helps clinicians understand the unique healthcare needs of the LGBTQ+ community, primarily in relation to end-of-life care. Topics include social and cultural background and challenges, healthcare disparities, advanced care planning, and strategies for reaching the community and improving quality of care.
Hypotension and role of physiotherapy in it

This particular slides consist of- what is hypotension,what are it's causes and it's effect on body, risk factors, symptoms,complications, diagnosis and role of physiotherapy in it.
This slide is very helpful for physiotherapy students and also for other medical and healthcare students.
Here is the summary of hypotension:
Hypotension, or low blood pressure, is when the pressure of blood circulating in the body is lower than normal or expected. It's only a problem if it negatively impacts the body and causes symptoms. Normal blood pressure is usually between 90/60 mmHg and 120/80 mmHg, but pressures below 90/60 are generally considered hypotensive.
Recently uploaded (20)
Innovative Minds France's Most Impactful Healthcare Leaders.pdf

Innovative Minds France's Most Impactful Healthcare Leaders.pdf
Can Allopathy and Homeopathy Be Used Together in India.pdf

Can Allopathy and Homeopathy Be Used Together in India.pdf
Tips for Pet Care in winters How to take care of pets.

Tips for Pet Care in winters How to take care of pets.
PET CT beginners Guide covers some of the underrepresented topics in PET CT

PET CT beginners Guide covers some of the underrepresented topics in PET CT
Michigan HealthTech Market Map 2024 with Policy Makers, Academic Innovation C...

Michigan HealthTech Market Map 2024 with Policy Makers, Academic Innovation C...
PrudentRx's Function in the Management of Chronic Illnesses

PrudentRx's Function in the Management of Chronic Illnesses
Stem Cell Solutions: Dr. David Greene's Path to Non-Surgical Cardiac Care

Stem Cell Solutions: Dr. David Greene's Path to Non-Surgical Cardiac Care
Deep Leg Vein Thrombosis (DVT): Meaning, Causes, Symptoms, Treatment, and Mor...

Deep Leg Vein Thrombosis (DVT): Meaning, Causes, Symptoms, Treatment, and Mor...
KEY Points of Leicester travel clinic In London doc.docx

KEY Points of Leicester travel clinic In London doc.docx
PrudentRx: A Resource for Patient Education and Engagement

PrudentRx: A Resource for Patient Education and Engagement
Feeding plate for a newborn with Cleft Palate.pptx

Feeding plate for a newborn with Cleft Palate.pptx
TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardso...

TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardso...
LGBTQ+ Adults: Unique Opportunities and Inclusive Approaches to Care

LGBTQ+ Adults: Unique Opportunities and Inclusive Approaches to Care
Japan medical device approval chart - Emergo
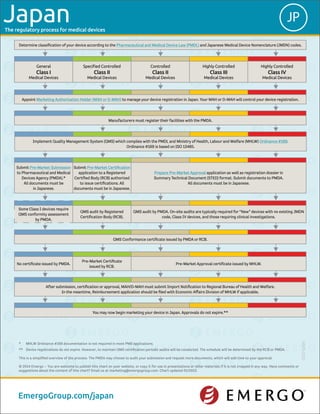
- 1. The regulatory process for medical devices You may now begin marketing your device in Japan. Approvals do not expire.** After submission, certification or approval, MAH/D-MAH must submit Import Notification to Regional Bureau of Health and Welfare. In the meantime, Reimbursement application should be filed with Economic Affairs Division of MHLW if applicable. No certificate issued by PMDA. Pre-Market Certificate issued by RCB. Pre-Market Approval certificate issued by MHLW. QMS Conformance certificate issued by PMDA or RCB. Some Class I devices require QMS conformity assessment by PMDA. QMS audit by Registered Certification Body (RCB). QMS audit by PMDA. On-site audits are typically required for “New” devices with no existing JMDN code, Class IV devices, and those requiring clinical investigations. Submit Pre-Market Submission to Pharmaceutical and Medical Devices Agency (PMDA).* All documents must be in Japanese. Submit Pre-Market Certification application to a Registered Certified Body (RCB) authorized to issue certifications. All documents must be in Japanese. Prepare Pre-Market Approval application as well as registration dossier in Summary Technical Document (STED) format. Submit documents to PMDA. All documents must be in Japanese. 5000-0115 * MHLW Ordinance #169 documentation is not required in most PMS applications. ** Device registrations do not expire. However, to maintain QMS certification periodic audits will be conducted. The schedule will be determined by the RCB or PMDA. This is a simplified overview of the process. The PMDA may choose to audit your submission and request more documents, which will add time to your approval. © 2014 Emergo – You are welcome to publish this chart on your website, or copy it for use in presentations or other materials if it is not cropped in any way. Have comments or suggestions about the content of this chart? Email us at marketing@emergogroup.com. Chart updated 01/2015. EmergoGroup.com/japan JPJapan Determine classification of your device according to the Pharmaceutical and Medical Device Law (PMDL) and Japanese Medical Device Nomenclature (JMDN) codes. General Class I Medical Devices Specified Controlled Class II Medical Devices Controlled Class II Medical Devices Highly Controlled Class III Medical Devices Highly Controlled Class IV Medical Devices Appoint Marketing Authorization Holder (MAH or D-MAH) to manage your device registration in Japan. Your MAH or D-MAH will control your device registration. Manufacturers must register their facilities with the PMDA. Implement Quality Management System (QMS) which complies with the PMDL and Ministry of Health, Labour and Welfare (MHLW) Ordinance #169. Ordinance #169 is based on ISO 13485.
- 2. The regulatory process for medical devices Device classification in Japan How long you should expect to wait after submission until approval is granted. (See note 1) Validity period for device registrations. (See note 2) Registration renewal should be started this far in advance. (See note 2) Complexity of the registration process for this classification. (See note 3) Overall cost of gaining regulatory approval. (See note 4) CLASS I General Medical Devices 1 month Does not expire 1 month Simple Complex Low High CLASS II Specified Controlled Medical Devices 3-5 months Does not expire 1 month Simple Complex Low High CLASS II Controlled Medical Devices 7-9 months Does not expire 1 month Simple Complex Low High CLASS III Highly Controlled Medical Devices 9-11 months Does not expire 1 month Simple Complex Low High CLASS IV Highly Controlled Medical Devices 13-16 months Does not expire 1 month Simple Complex Low High JPJapan EmergoGroup.com/japan NOTE 1: The time frames shown above are typical for the majority of medical device submissions but assume that your device does not contain animal tissue, medicinal substances or employ entirely novel technology. Your length of approval will depend on the quality and completeness of your technical documentation and how much time you take to address additional information requests from authorities after submission. YOUR SUBMISSION(S) MAY TAKE MORE TIME THAN WHAT IS SHOWN ABOVE. NOTE 2: Device registration does not expire, however you must pay Foreign Manufacturer Registration (FMR) renewal fees. Failure to renew FMR will result in your permission to market your device being revoked. NOTE 3: Our rating of the complexity of the registration process is based on our experience and the opinion of nearly 1,000 QA/RA professionals worldwide who were asked to rate the difficulty of registering a device in each country in January 2014. The European CE Marking process is considered the mid-point to which all other markets are compared. NOTE 4: Low = Less than US$5000; Midpoint = US$15000-$30000: High = More than US$50000. Overall cost includes registration application fees, product testing, in- country representation, submission preparation consulting and translation of registration documents but not IFU. Does not include cost of implementing or auditing a quality management system compliant with ISO 13485 or US FDA 21 CFR Part 820, if applicable. © 2014 Emergo Group – You are welcome to publish this chart on your website, or copy it for use in presentations or other materials if it is not cropped in any way. Have comments or suggestions about the content of this chart? Email us at marketing@emergogroup.com. Chart updated 01/2015.
