Europe CE Marking for medical devices under new MDR
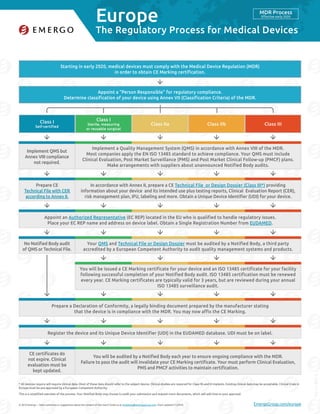
- 1. Europe © 2016 Emergo – Have comments or suggestions about the content of this chart? Email us at marketing@emergogroup.com. Chart updated 11/2016. EmergoGroup.com/europe Starting in early 2020, medical devices must comply with the Medical Device Regulation (MDR) in order to obtain CE Marking certification. Appoint a “Person Responsible” for regulatory compliance. Determine classification of your device using Annex VII (Classification Criteria) of the MDR. Appoint an Authorized Representative (EC REP) located in the EU who is qualified to handle regulatory issues. Place your EC REP name and address on device label. Obtain a Single Registration Number from EUDAMED. Prepare a Declaration of Conformity, a legally binding document prepared by the manufacturer stating that the device is in compliance with the MDR. You may now affix the CE Marking. Register the device and its Unique Device Identifier (UDI) in the EUDAMED database. UDI must be on label. In accordance with Annex II, prepare a CE Technical File or Design Dossier (Class III*) providing information about your device and its intended use plus testing reports, Clinical Evaluation Report (CER), risk management plan, IFU, labeling and more. Obtain a Unique Device Identifier (UDI) for your device. You will be audited by a Notified Body each year to ensure ongoing compliance with the MDR. Failure to pass the audit will invalidate your CE Marking certificate. Your must perform Clinical Evaluation, PMS and PMCF activities to maintain certification. Implement a Quality Management System (QMS) in accordance with Annex VIII of the MDR. Most companies apply the EN ISO 13485 standard to achieve compliance. Your QMS must include Clinical Evaluation, Post Market Surveillance (PMS) and Post Market Clinical Follow-up (PMCF) plans. Make arrangements with suppliers about unannounced Notified Body audits. You will be issued a CE Marking certificate for your device and an ISO 13485 certificate for your facility following successful completion of your Notified Body audit. ISO 13485 certification must be renewed every year. CE Marking certificates are typically valid for 3 years, but are reviewed during your annual ISO 13485 surveillance audit. Your QMS and Technical File or Design Dossier must be audited by a Notified Body, a third party accredited by a European Competent Authority to audit quality management systems and products. Prepare CE Technical File with CER according to Annex II. CE certificates do not expire. Clinical evaluation must be kept updated. Class I Self-certified Class IIa Class I Sterile, measuring or reusable surgical Class IIb Class III 5168-1116 The Regulatory Process for Medical Devices MDR Process Effective early 2020 * All devices require will require clinical data. Most of these data should refer to the subject device. Clinical studies are required for Class IIb and III implants. Existing clinical data may be acceptable. Clinical trials in Europe must be pre-approved by a European Competent Authority. This is a simplified overview of the process. Your Notified Body may choose to audit your submission and request more documents, which will add time to your approval. Implement QMS but Annex VIII compliance not required. No Notified Body audit of QMS or Technical File.
- 2. Europe Notes 1. The MDR was not released until mid-2016. Emergo expects there will be far more demand for recertification services between now and 2020 than the existing pool of Notified Bodies can handle. We expect this will lead to significantly longer review times than the 3-9 month approval timelines experienced under the MDD. Your length of approval will also depend on the quality and completeness of your technical documentation, more specifically your clinical data, and how much time you take to address additional information requests from authorities after submission. 2. CE Marking certificates are typically valid for a maximum of 5 years, but are generally reviewed annually at the same time as the ISO 13485 surveillance audit. They remain valid as long as you do not make changes to the device, intended use or indications for use and you perform adequate clinical evaluation activities, including PMS and PMCF. Failure to pass your annual audit could invalidate your CE Marking certificate. 3. Most CE Marking certificates are valid for a maximum of 5 years, and you do not need to “re-register” your device in Europe. However, your Notified Body will conduct an annual compliance audit and could invalidate your device CE certificate if you are found to be out of compliance. Your Notified Body will reissue your CE certificate every five years. Annual and renewal audits require careful planning. We recommend starting the preparations for your annual audit no later than the time specified above. Please consult with your regulatory expert well before this suggested time to avoid any lapse in your registration. 4. Our rating of the complexity of the registration process is based on our extensive experience and the opinion of 1,000 QA/RA professionals worldwide who were asked to rate the difficulty of registering a device in each country. 5. Prices in US Dollars for a single device. 1 = Less than $5,000; 2 = $10,000 - $15,000; 3 = $15,000 - $30,000; 4 = $30,000 - $50,000; 5 = $50,000 or more. Estimated cost includes registration application fees, in-country representation, Notified Body audit fees, submission preparation consulting and translation of documents, as required. It is important to note that these estimates are based on the existing process under the Medical Devices Directive (93/42/EEC). As more stringent requirements are put into place with the MDR, we expect overall CE compliance costs to rise. This remains to be seen so please only use these number for very rough planning purposes. Costs assume you already have approval for your device in the United States, Canada, Australia or Japan. Cost do NOT include product testing, clinical trials, or ISO 13485 implementation, if applicable. These costs are generally shared across many markets and thus not specifically attributed to a European CE submission. EmergoGroup.com/europe Device classification in Europe Class I Self-certified Class I Sterile, measuring or reusable surgical Class IIa Class IIb Class III How long you should expect to wait after submission until approval is granted.1 See note 1* See note 1 See note 1 See note 1 See note 1 Validity period for CE Marking certificate.2 Does not expire 5 years 5 years 5 years 5 years Registration renewal should be started this far in advance.3 Not applicible 6 months 6 months 6 months 6 months Complexity of the registration process for this classification.4 Estimated cost (USD) of gaining regulatory approval .5 5168-1116 © 2016 Emergo – Have comments or suggestions about the content of this table? Email us at marketing@emergogroup.com. Table updated 11/2016. Time, Cost, and Complexity of Registration Simple Complex Low High Simple Complex Low High Simple Complex Low High Simple Complex Low High Simple Complex Low High Class I devices which are not provided sterile, do not have a measuring function or are reusable surgical instruments can be self-certified (self-declared). Entering the required data into EUDAMED will be sufficient to allow placement on the market in Europe. However, this can only be done if the manufacturer has obtained a Single Registration Number to identify the company, and a UDI to identify the device. Currently the timelines for implementation of these databases is unknown. This is a simplified overview of the process. Your Notified Body may choose to audit your submission and request more documents, which will add time to your approval. * 5 55 1 2 2 3 3 4 4 MDR Process Effective early 2020
