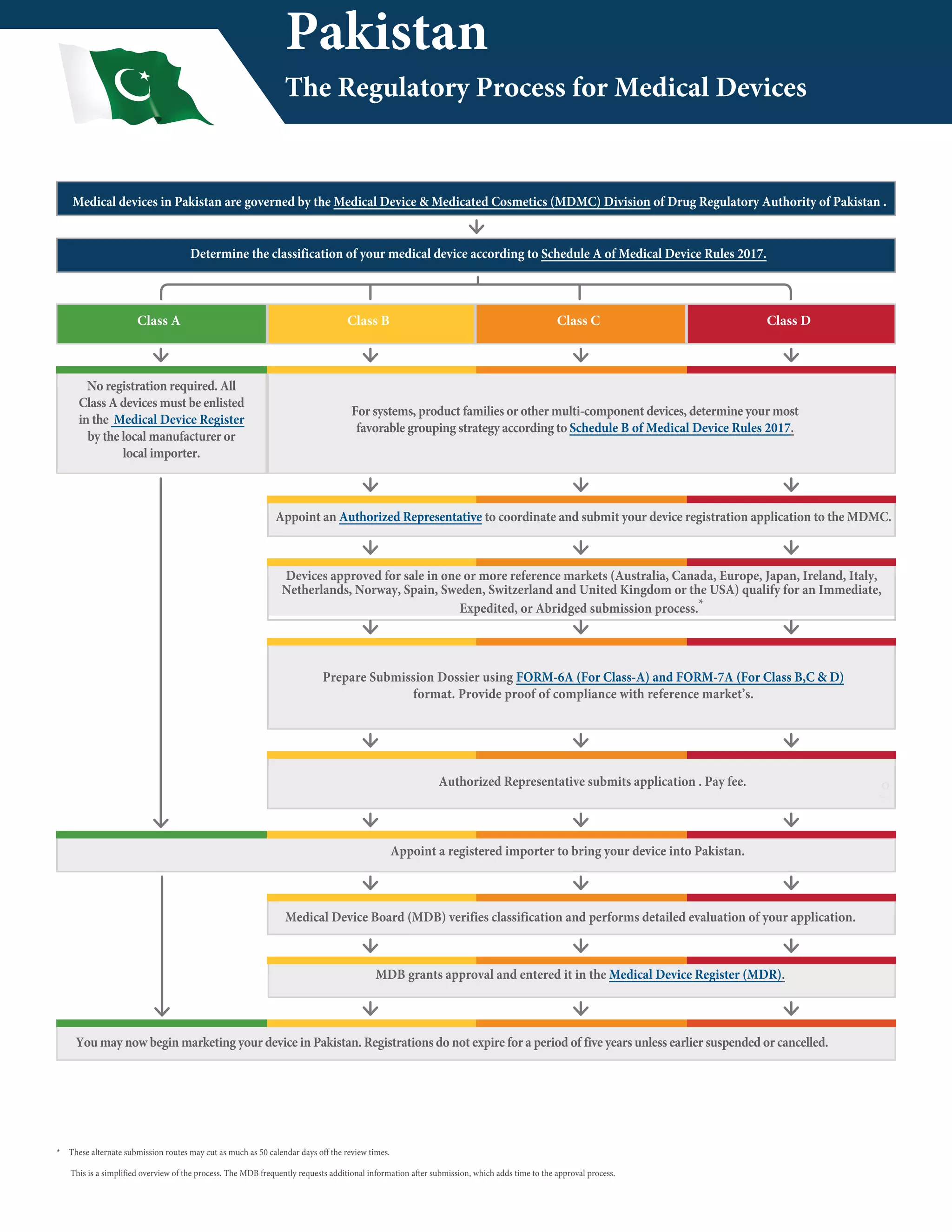
Medical devices in Pakistan are regulated by the Medical Device & Medicated Cosmetics Division and must be classified according to the Medical Device Rules of 2017. The regulatory process involves appointing a registered importer, determining the classification, and having an authorized representative submit an application dossier and fees to the Medical Devices Board for approval. Approval times range from less than two months for Class A local manufacturer devices to 8-10 months for Class D imported devices not from reference countries.