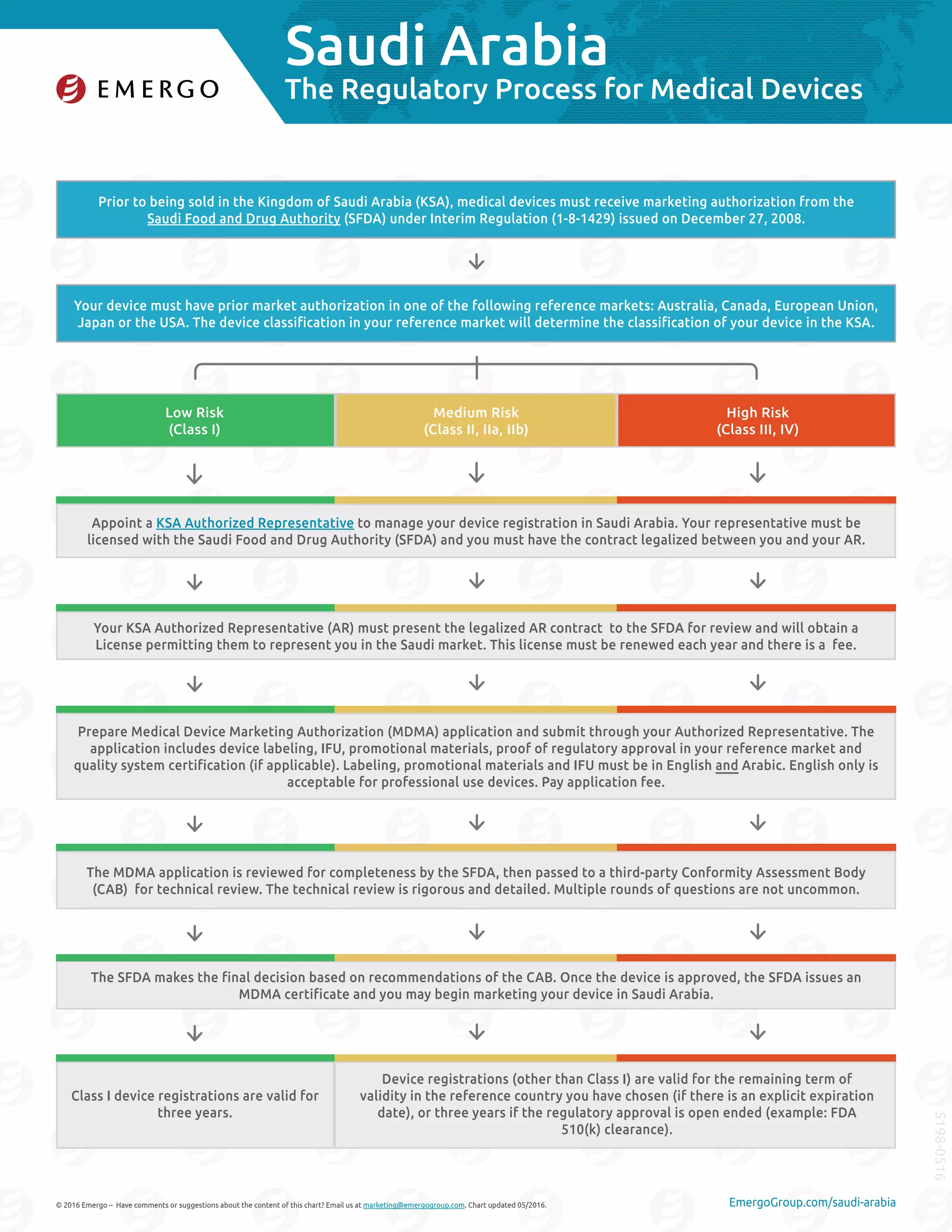
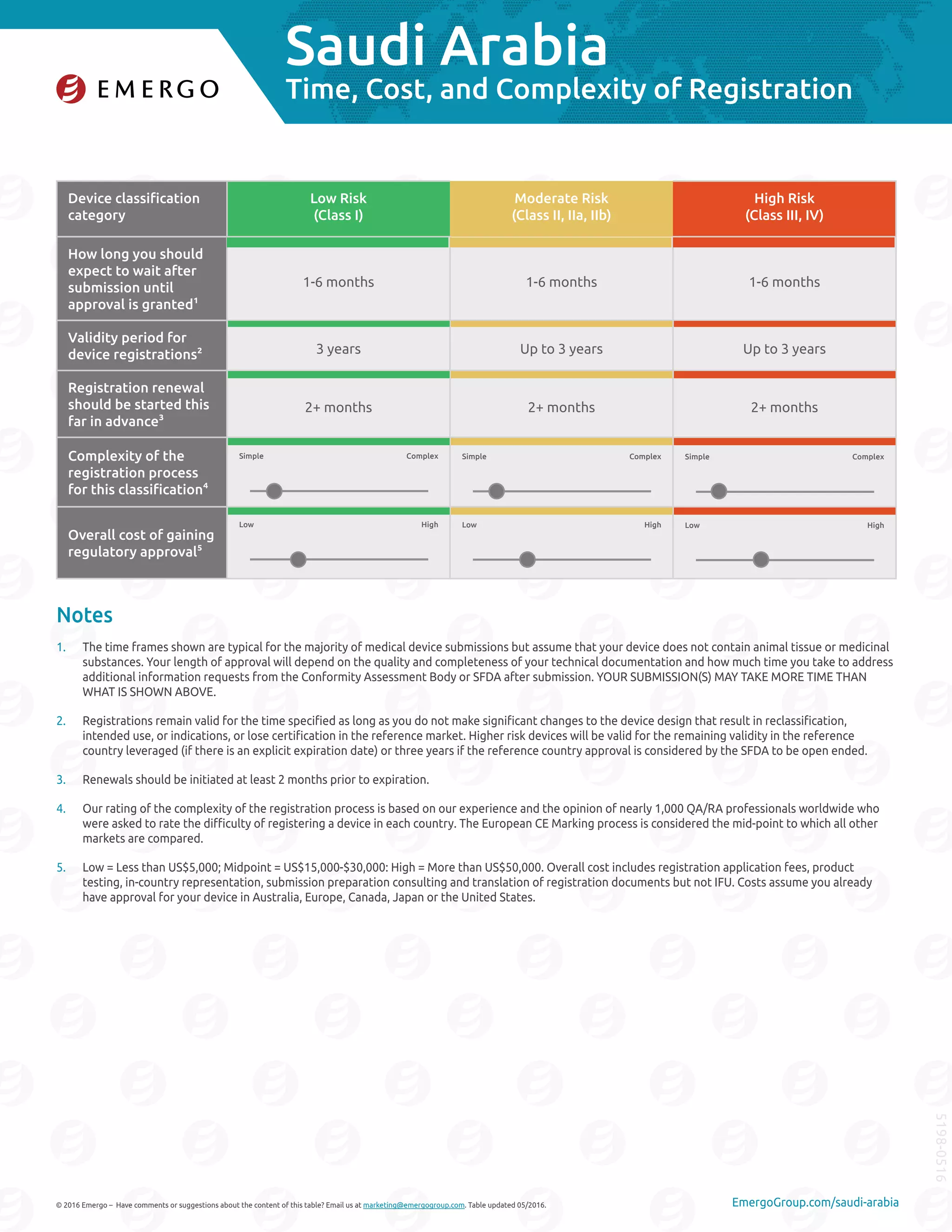
To market medical devices in Saudi Arabia, devices must receive marketing authorization from the Saudi Food and Drug Authority (SFDA). The SFDA application is reviewed for completeness then sent to a third party for technical review, which can involve multiple rounds of questions. Once approved, the SFDA issues a certificate allowing marketing in Saudi Arabia. Appointing an authorized local representative is also required to manage the registration process. Overall, registration times range from 1-6 months but costs and complexity vary depending on the device's risk classification.