India medical device regulatory process
•
4 likes•6,094 views
The regulatory framework for medical devices in India is based on drug regulations under the Drugs and Cosmetics Act of 1940 and Drugs and Cosmetics Rules of 1945. The Drug Controller General of India (DCGI) within the Central Drugs Standard Control Organization (CDSCO) regulates medical devices and IVDs. Currently only a limited number of medical device and IVD products require registration in India, including ablation devices, dental implants, and hernia mesh. The registration process for notified devices can take 9-12 months and involves appointing an authorized agent, compiling an application, and obtaining CDSCO approval.
Report
Share
Report
Share
Download to read offline
Recommended
Medical device approval chart for Russia - EMERGO

The regulatory process for medical devices in Russia involves classifying the device, appointing an authorized representative, submitting a registration dossier including technical documentation and clinical data to Roszdravnadzor (RZN), addressing any requests for additional information, and ultimately receiving a registration certificate to market the device in Russia. The process can take 6-12 months depending on the device class.
Japan medical device approval chart - Emergo 

The regulatory process for medical devices in Japan involves several key steps:
1) Classifying the device and appointing a Marketing Authorization Holder who will manage the registration process.
2) Implementing a Quality Management System that complies with relevant regulations.
3) Submitting an application to either the Pharmaceuticals and Medical Devices Agency or a Registered Certification Body, depending on the device class.
4) Undergoing an audit and receiving the appropriate certificate before marketing the device in Japan. Device registrations do not expire but must be renewed periodically.
US FDA medical device approval chart - Emergo 

Simple one page chart shows the US FDA medical device approval process for Class 1, 2 and 3 devices. Very easy to understand.
Canada medical device approval chart - EMERGO

1. To market medical devices in Canada, manufacturers must classify their device according to regulations, implement an ISO 13485 quality management system with additional Canadian requirements, and obtain ISO 13485 certification from an accredited registrar.
2. Manufacturers then prepare license applications, supporting documents, and pay fees, with process times ranging from 1-8 months depending on device class.
3. Licenses must be renewed annually by meeting regulatory requirements and paying fees to avoid revocation.
Saudi arabia medical device regulatory process

To market medical devices in Saudi Arabia, devices must receive marketing authorization from the Saudi Food and Drug Authority (SFDA). The SFDA application is reviewed for completeness then sent to a third party for technical review, which can involve multiple rounds of questions. Once approved, the SFDA issues a certificate allowing marketing in Saudi Arabia. Appointing an authorized local representative is also required to manage the registration process. Overall, registration times range from 1-6 months but costs and complexity vary depending on the device's risk classification.
Taiwan medical device registration and approval chart - EMERGO

Taiwan regulates medical devices through the Pharmaceutical Affairs Act and Regulations for Governing the Management of Medical Devices. The process involves classifying the device, appointing a Taiwan agent, preparing quality system documentation for submission, and obtaining approval. Device classification and complexity of approval requirements vary, with Class I generally having the simplest process taking 1-2 months, Class II taking 10-12 months, and Class III taking 10-12 months and requiring a committee review.
Regulatory requirements for CE CERTIFICATION of Medical Devices in European U...

The EU introduced the CE marking scheme to make trade easier and cheaper between EU countries. It means that a manufacturer claims that their product conforms to the minimum legal requirements for health and safety as laid down in EU directives. In addition, it may be considered a benefit that by implementing the requirements, the product will be safer for the user and this may also reduce damage and liability claims. Additional benefits may include your product being made safer for end-users. The EU introduced the CE marking scheme to make trade easier and cheaper between EU countries. It means that a manufacturer claims that their product conforms to the minimum legal requirements for health and safety as laid down in EU directives. If you manufacture or import a product which falls within the scope of one or more of the New Approach Directives and wish to place your product on the market in any of the member’s states of the European Economic Area (EEA), then you must apply CE marking to your product against the essential requirements of all these applicable directives.
Key Words: European Union, CE marking, New Approach Directives, EEA, Regulatory requirements.
Europe IVD medical registration and approval chart - EMERGO

The document summarizes the regulatory process for in vitro diagnostic devices (IVDs) in Europe under the In Vitro Diagnostic Directive (98/79/EC). It outlines the classification of IVDs, requirements for quality management systems and technical files, roles of notified bodies and authorized representatives, and timelines and costs associated with the approval process depending on the IVD classification. The process can take from 3-5 months for self-certified IVDs to 9-12 months for list A IVDs and involves implementing quality systems, obtaining notified body audits, and registering with authorities.
Recommended
Medical device approval chart for Russia - EMERGO

The regulatory process for medical devices in Russia involves classifying the device, appointing an authorized representative, submitting a registration dossier including technical documentation and clinical data to Roszdravnadzor (RZN), addressing any requests for additional information, and ultimately receiving a registration certificate to market the device in Russia. The process can take 6-12 months depending on the device class.
Japan medical device approval chart - Emergo 

The regulatory process for medical devices in Japan involves several key steps:
1) Classifying the device and appointing a Marketing Authorization Holder who will manage the registration process.
2) Implementing a Quality Management System that complies with relevant regulations.
3) Submitting an application to either the Pharmaceuticals and Medical Devices Agency or a Registered Certification Body, depending on the device class.
4) Undergoing an audit and receiving the appropriate certificate before marketing the device in Japan. Device registrations do not expire but must be renewed periodically.
US FDA medical device approval chart - Emergo 

Simple one page chart shows the US FDA medical device approval process for Class 1, 2 and 3 devices. Very easy to understand.
Canada medical device approval chart - EMERGO

1. To market medical devices in Canada, manufacturers must classify their device according to regulations, implement an ISO 13485 quality management system with additional Canadian requirements, and obtain ISO 13485 certification from an accredited registrar.
2. Manufacturers then prepare license applications, supporting documents, and pay fees, with process times ranging from 1-8 months depending on device class.
3. Licenses must be renewed annually by meeting regulatory requirements and paying fees to avoid revocation.
Saudi arabia medical device regulatory process

To market medical devices in Saudi Arabia, devices must receive marketing authorization from the Saudi Food and Drug Authority (SFDA). The SFDA application is reviewed for completeness then sent to a third party for technical review, which can involve multiple rounds of questions. Once approved, the SFDA issues a certificate allowing marketing in Saudi Arabia. Appointing an authorized local representative is also required to manage the registration process. Overall, registration times range from 1-6 months but costs and complexity vary depending on the device's risk classification.
Taiwan medical device registration and approval chart - EMERGO

Taiwan regulates medical devices through the Pharmaceutical Affairs Act and Regulations for Governing the Management of Medical Devices. The process involves classifying the device, appointing a Taiwan agent, preparing quality system documentation for submission, and obtaining approval. Device classification and complexity of approval requirements vary, with Class I generally having the simplest process taking 1-2 months, Class II taking 10-12 months, and Class III taking 10-12 months and requiring a committee review.
Regulatory requirements for CE CERTIFICATION of Medical Devices in European U...

The EU introduced the CE marking scheme to make trade easier and cheaper between EU countries. It means that a manufacturer claims that their product conforms to the minimum legal requirements for health and safety as laid down in EU directives. In addition, it may be considered a benefit that by implementing the requirements, the product will be safer for the user and this may also reduce damage and liability claims. Additional benefits may include your product being made safer for end-users. The EU introduced the CE marking scheme to make trade easier and cheaper between EU countries. It means that a manufacturer claims that their product conforms to the minimum legal requirements for health and safety as laid down in EU directives. If you manufacture or import a product which falls within the scope of one or more of the New Approach Directives and wish to place your product on the market in any of the member’s states of the European Economic Area (EEA), then you must apply CE marking to your product against the essential requirements of all these applicable directives.
Key Words: European Union, CE marking, New Approach Directives, EEA, Regulatory requirements.
Europe IVD medical registration and approval chart - EMERGO

The document summarizes the regulatory process for in vitro diagnostic devices (IVDs) in Europe under the In Vitro Diagnostic Directive (98/79/EC). It outlines the classification of IVDs, requirements for quality management systems and technical files, roles of notified bodies and authorized representatives, and timelines and costs associated with the approval process depending on the IVD classification. The process can take from 3-5 months for self-certified IVDs to 9-12 months for list A IVDs and involves implementing quality systems, obtaining notified body audits, and registering with authorities.
Medical device regulation US, European Union and India

This document summarizes medical device regulations in the United States, European Union, and India. It discusses how medical devices are classified based on risk in each region, with Class I being lowest risk and Class III being highest. The regulatory approval processes for medical devices in each location are also outlined, including applying for certification marks like the FDA clearance in the US or CE Marking in the EU. Finally, the document provides statistics on the global market share of the medical device industry and references used.
Brazil medical device registration and approval chart - EMERGO

Brazil has a classification system for medical devices with four classes - I, II, III, and IV. Class I and II devices require a simplified registration called a Cadastro, while Class III and IV devices require a more rigorous registration called a Registro. To register a device, a company must appoint a Brazil Registration Holder to submit the application to ANVISA and obtain necessary approvals. Certain devices may also require certification from inspection agency INMETRO. The registration process can take 1-3 months for Classes I and II or 8-15 months for Classes III and IV, though Class III and IV can take over 4 years if additional inspections are required.
Medical devices

To comprehend the regulatory requirements to import medical Medical devices and authorization procedures in regulated markets of the United States and Australia
China medical device approval chart - EMERGO

1. The document outlines the regulatory process for medical devices in China, including classification of devices and the approval process for Class I, II, and III devices.
2. Class I devices have the simplest approval process, requiring only an administrative review with no submission fees. Class II and III devices require more extensive technical documentation, testing in China, and clinical evaluations.
3. The approval process can take 12-22 months for Class II and III devices and involves appointing a Chinese agent, submitting documentation and application materials in Chinese, testing, and a review by the China Food and Drug Administration before a registration certificate is issued.
Europe CE Marking for medical devices under new MDR

Starting in early 2020, medical devices seeking CE Marking certification in Europe must comply with the new Medical Device Regulation (MDR). This will require appointing personnel responsible for regulatory compliance, classifying the device, appointing an Authorized Representative located in the EU, including labeling with this information, and obtaining a Single Registration Number. Manufacturers must also prepare technical documentation, implement a quality management system, and undergo annual audits by a Notified Body to maintain certification.
Medical device approval chart for Mexico - Emergo 

In Mexico, medical devices and in-vitro diagnostic devices are regulated by COFEPRIS, a division of the Mexican Ministry of Health. The regulatory process involves classifying the device, appointing a Mexico Registration Holder representative, preparing a registration dossier in Spanish including documentation of quality management and technical specifications, and submitting the application to COFEPRIS for review. Approval time ranges from 1-10 months depending on the device class and review process. Registrations are valid for 5 years and must be renewed in advance of the expiration date.
The EU’s Medical Device Regulation

The EU’s medical device regulation
Medical device manufacturers seeking market access
to the European Union (EU) will soon face major changes
in the EU’s decades-old regulatory framework. The EU’s
Medical Device Regulation (MDR) was officially published
on 5 May 2017 and came into force on 25 May 2017.
The MDR will replace the EU’s current Medical Device
Directive (93/42/EEC) and the EU’s Directive on active
implantable medical devices (90/385/EEC).
South Korea medical device approval chart - Emergo 

The regulatory process for medical devices in South Korea requires manufacturers to determine the classification of their device and submit the appropriate registration application and supporting documentation to the Ministry of Food and Drug Safety (MFDS). For new devices without a predicate, manufacturers must submit clinical data as part of a Clinical Data Review. Class II devices considered "Special Equivalent" have an expediated review process without a Technical File requirement. Prior international testing may satisfy some requirements but additional Korea-specific testing is often necessary. The MFDS may request additional information or audit submissions, extending approval timelines.
The European Medical Device Regulations - analysis of the final text

Following the publication of the new European Medical Device Regulations by the European Commission, we offer you our analysis of the final text.
medical device regulatory approval in USA

The document discusses the approval process for medical devices in the United States, including an overview of the classification system for medical devices (Class I, II, III), the requirements for each class (e.g. 510(k) notification or premarket approval), and the components required for a 510(k) premarket notification application to the FDA.
Japan Medical Device Regulatory Approval Process

Before deciding to sell your device in the Japanese market, it is important to understand how the regulations apply to your device, which steps to take, and what resources are required to complete the process. In this presentation, Ann Marie Boullie, Vice President of Business Development for EMERGO, outlines some of the most complex aspects of the Japanese registration process, including:
JMDN codes: device classification and predicates
Clinical data requirements and PMDA pre-submission meetings
Registration routes (Todokede, Ninsho, Shonin)
QMS (Ordinance 169) requirements
Role of the Marketing Authorization Holder (MAH)
Regulatory Approval Process for Medical Devices in EU - Presentation by Aksha...

A presentation on Regulatory Approval Process for Medical Devices in European Union that explains in brief about the various aspects including the EU Medical Device Directives, Classifications, CE Certification, Medical Device Registration & Timelines. This was presented as a part of curriculum by Akshay Anand in JSS College of Pharmacy, Mysuru during January 2015
Medical Device Regulations - 510(k) Process

This document provides an overview of the 510(k) premarket notification process required by the FDA for medical devices. It explains that the 510(k) process requires manufacturers to demonstrate that new devices are substantially equivalent to existing legally marketed predicate devices. The document outlines the key requirements for submitting a 510(k), including when one is necessary, who must submit one, what information must be included, and scenarios where a 510(k) is not required. It also provides details on the review process and requirements for devices that are cleared through the 510(k) pathway.
Regulation of medical device in japan

Medical Devices registration in Japan , quality system requirements and evaluation and investigation of medical devices in regulated countries ASEAN, China JAPAN and WHO regulations. quality and ethical considerations regulatory and documentation requirements for marketing medical devices and IVDs in regulated countries.
Medical Devices Regulation (MDR) 2017/745 - Clinical investigations

Medical Devices Regulation (MDR) 2017/745 - Clinical investigations
See more https://www.aretezoe.com/medical-devices
CE marking and CE certification

CE marking and CE certification what is it why you need it who can apply
CE marking certification for medical devices. Medical Device Regulations. It Is Easy To Make Mistakes In The Regulatory Process That Can Delay.
Visit: http://www.meddevicecorp.com/
Regulatory requirement and approval procedure for medical devices

regulation of medical devices in india, licensing requirement and their procedure by amended medical device rule 2017
7 Steps - How to Get a CE Marking Certification for Medical Devices?

The document outlines the 7 steps to obtain CE marking certification for a medical device: 1) Classify the device, 2) Identify relevant standards and regulations, 3) Compile technical documentation and testing results, 4) Appoint a European authorized representative if located outside the EU, 5) Obtain certification from a notified body for class II/III devices or self-certify for class I, 6) Affix the CE marking, and 7) Comply with any national requirements. The service described can assist manufacturers with all aspects of the certification process.
Medical device regulations in india

A brief introduction on medical device , how iit is regulated in india,its marketing authorization,classification, notified devices,import of device and registration process, fnctions of medical device department.
Regulation of Medical Devices in US

The document discusses the regulation of medical devices in the United States. It begins by defining what constitutes a medical device according to the Code of Federal Regulations. It then outlines the key regulatory bodies that oversee medical devices, including the Center for Devices and Radiological Health and the Office of Combination Products. The document provides an overview of the classification system for medical devices and the different regulatory pathways for approval, including 510(k) premarket notification, investigational device exemptions, premarket approval, and humanitarian device exemption. It also summarizes the key requirements and processes for each approval pathway.
Medical Device Registration in India 

Rules and regulations for medical device registration, forms and fees required, types of medical devices to be registered under CDSCO India.
Indian market overview
India’s medical device regulatory structure
Categories of regulated medical devices in India
No Objection Certificates
Device Registration Certificate applications
Import Licensing
India Authorized Agent selection
Costs and Timelines
Developing Regulatory Issues in India
Shruthi seminar final ppt (1)

Medical device and Patency
By MDD 93/42
Any devices including an instrument, apparatus, appliance, implant, material or other article, whether used alone or in combination, including a software or an accessory, intended by its manufacturer to be used specially for human beings or animals which does not achieve the primary intended action in or on human body or animals by any pharmacological or immunological or metabolic means, but which may assist in its intended function by such means for one or more of the specific purposes of ―
diagnosis, prevention, monitoring, treatment or alleviation of any disease or disorder;
diagnosis, monitoring, treatment, alleviation or assistance for, any injury or disability;
investigation, replacement or modification or support of the anatomy or of a physiological process;
supporting or sustaining life;
disinfection of medical devices; and
control of conception.
What is 510(k) ?
A 510(k) is a premarket submission made to FDA to demonstrate
that the device to be marketed is as safe and effective, that is,
substantially equivalent, to a legally marketed device
(section 513(i)(1)(a) FD&C act).
Medical Devices are notified as DRUGS under Drugs & Cosmetics Act. Section 3 (b) (iv) defines,
Medical Devices as “Devices intended for internal or external use in the diagnosis, treatment, mitigation or prevention of disease or disorder in human beings or animals”
India had no regulation for medical devices in place prior to 2005.
The import, manufacturing, distribution and Sale of medical devices in India are overseen by the Drugs and Cosmetics Act (1940) and Rules (1945)
Control and inspection are carried out by the CDSCO, state drug controllers and central/state laboratories.
More Related Content
What's hot
Medical device regulation US, European Union and India

This document summarizes medical device regulations in the United States, European Union, and India. It discusses how medical devices are classified based on risk in each region, with Class I being lowest risk and Class III being highest. The regulatory approval processes for medical devices in each location are also outlined, including applying for certification marks like the FDA clearance in the US or CE Marking in the EU. Finally, the document provides statistics on the global market share of the medical device industry and references used.
Brazil medical device registration and approval chart - EMERGO

Brazil has a classification system for medical devices with four classes - I, II, III, and IV. Class I and II devices require a simplified registration called a Cadastro, while Class III and IV devices require a more rigorous registration called a Registro. To register a device, a company must appoint a Brazil Registration Holder to submit the application to ANVISA and obtain necessary approvals. Certain devices may also require certification from inspection agency INMETRO. The registration process can take 1-3 months for Classes I and II or 8-15 months for Classes III and IV, though Class III and IV can take over 4 years if additional inspections are required.
Medical devices

To comprehend the regulatory requirements to import medical Medical devices and authorization procedures in regulated markets of the United States and Australia
China medical device approval chart - EMERGO

1. The document outlines the regulatory process for medical devices in China, including classification of devices and the approval process for Class I, II, and III devices.
2. Class I devices have the simplest approval process, requiring only an administrative review with no submission fees. Class II and III devices require more extensive technical documentation, testing in China, and clinical evaluations.
3. The approval process can take 12-22 months for Class II and III devices and involves appointing a Chinese agent, submitting documentation and application materials in Chinese, testing, and a review by the China Food and Drug Administration before a registration certificate is issued.
Europe CE Marking for medical devices under new MDR

Starting in early 2020, medical devices seeking CE Marking certification in Europe must comply with the new Medical Device Regulation (MDR). This will require appointing personnel responsible for regulatory compliance, classifying the device, appointing an Authorized Representative located in the EU, including labeling with this information, and obtaining a Single Registration Number. Manufacturers must also prepare technical documentation, implement a quality management system, and undergo annual audits by a Notified Body to maintain certification.
Medical device approval chart for Mexico - Emergo 

In Mexico, medical devices and in-vitro diagnostic devices are regulated by COFEPRIS, a division of the Mexican Ministry of Health. The regulatory process involves classifying the device, appointing a Mexico Registration Holder representative, preparing a registration dossier in Spanish including documentation of quality management and technical specifications, and submitting the application to COFEPRIS for review. Approval time ranges from 1-10 months depending on the device class and review process. Registrations are valid for 5 years and must be renewed in advance of the expiration date.
The EU’s Medical Device Regulation

The EU’s medical device regulation
Medical device manufacturers seeking market access
to the European Union (EU) will soon face major changes
in the EU’s decades-old regulatory framework. The EU’s
Medical Device Regulation (MDR) was officially published
on 5 May 2017 and came into force on 25 May 2017.
The MDR will replace the EU’s current Medical Device
Directive (93/42/EEC) and the EU’s Directive on active
implantable medical devices (90/385/EEC).
South Korea medical device approval chart - Emergo 

The regulatory process for medical devices in South Korea requires manufacturers to determine the classification of their device and submit the appropriate registration application and supporting documentation to the Ministry of Food and Drug Safety (MFDS). For new devices without a predicate, manufacturers must submit clinical data as part of a Clinical Data Review. Class II devices considered "Special Equivalent" have an expediated review process without a Technical File requirement. Prior international testing may satisfy some requirements but additional Korea-specific testing is often necessary. The MFDS may request additional information or audit submissions, extending approval timelines.
The European Medical Device Regulations - analysis of the final text

Following the publication of the new European Medical Device Regulations by the European Commission, we offer you our analysis of the final text.
medical device regulatory approval in USA

The document discusses the approval process for medical devices in the United States, including an overview of the classification system for medical devices (Class I, II, III), the requirements for each class (e.g. 510(k) notification or premarket approval), and the components required for a 510(k) premarket notification application to the FDA.
Japan Medical Device Regulatory Approval Process

Before deciding to sell your device in the Japanese market, it is important to understand how the regulations apply to your device, which steps to take, and what resources are required to complete the process. In this presentation, Ann Marie Boullie, Vice President of Business Development for EMERGO, outlines some of the most complex aspects of the Japanese registration process, including:
JMDN codes: device classification and predicates
Clinical data requirements and PMDA pre-submission meetings
Registration routes (Todokede, Ninsho, Shonin)
QMS (Ordinance 169) requirements
Role of the Marketing Authorization Holder (MAH)
Regulatory Approval Process for Medical Devices in EU - Presentation by Aksha...

A presentation on Regulatory Approval Process for Medical Devices in European Union that explains in brief about the various aspects including the EU Medical Device Directives, Classifications, CE Certification, Medical Device Registration & Timelines. This was presented as a part of curriculum by Akshay Anand in JSS College of Pharmacy, Mysuru during January 2015
Medical Device Regulations - 510(k) Process

This document provides an overview of the 510(k) premarket notification process required by the FDA for medical devices. It explains that the 510(k) process requires manufacturers to demonstrate that new devices are substantially equivalent to existing legally marketed predicate devices. The document outlines the key requirements for submitting a 510(k), including when one is necessary, who must submit one, what information must be included, and scenarios where a 510(k) is not required. It also provides details on the review process and requirements for devices that are cleared through the 510(k) pathway.
Regulation of medical device in japan

Medical Devices registration in Japan , quality system requirements and evaluation and investigation of medical devices in regulated countries ASEAN, China JAPAN and WHO regulations. quality and ethical considerations regulatory and documentation requirements for marketing medical devices and IVDs in regulated countries.
Medical Devices Regulation (MDR) 2017/745 - Clinical investigations

Medical Devices Regulation (MDR) 2017/745 - Clinical investigations
See more https://www.aretezoe.com/medical-devices
CE marking and CE certification

CE marking and CE certification what is it why you need it who can apply
CE marking certification for medical devices. Medical Device Regulations. It Is Easy To Make Mistakes In The Regulatory Process That Can Delay.
Visit: http://www.meddevicecorp.com/
Regulatory requirement and approval procedure for medical devices

regulation of medical devices in india, licensing requirement and their procedure by amended medical device rule 2017
7 Steps - How to Get a CE Marking Certification for Medical Devices?

The document outlines the 7 steps to obtain CE marking certification for a medical device: 1) Classify the device, 2) Identify relevant standards and regulations, 3) Compile technical documentation and testing results, 4) Appoint a European authorized representative if located outside the EU, 5) Obtain certification from a notified body for class II/III devices or self-certify for class I, 6) Affix the CE marking, and 7) Comply with any national requirements. The service described can assist manufacturers with all aspects of the certification process.
Medical device regulations in india

A brief introduction on medical device , how iit is regulated in india,its marketing authorization,classification, notified devices,import of device and registration process, fnctions of medical device department.
Regulation of Medical Devices in US

The document discusses the regulation of medical devices in the United States. It begins by defining what constitutes a medical device according to the Code of Federal Regulations. It then outlines the key regulatory bodies that oversee medical devices, including the Center for Devices and Radiological Health and the Office of Combination Products. The document provides an overview of the classification system for medical devices and the different regulatory pathways for approval, including 510(k) premarket notification, investigational device exemptions, premarket approval, and humanitarian device exemption. It also summarizes the key requirements and processes for each approval pathway.
What's hot (20)
Medical device regulation US, European Union and India

Medical device regulation US, European Union and India
Brazil medical device registration and approval chart - EMERGO

Brazil medical device registration and approval chart - EMERGO
Europe CE Marking for medical devices under new MDR

Europe CE Marking for medical devices under new MDR
South Korea medical device approval chart - Emergo 

South Korea medical device approval chart - Emergo
The European Medical Device Regulations - analysis of the final text

The European Medical Device Regulations - analysis of the final text
Regulatory Approval Process for Medical Devices in EU - Presentation by Aksha...

Regulatory Approval Process for Medical Devices in EU - Presentation by Aksha...
Medical Devices Regulation (MDR) 2017/745 - Clinical investigations

Medical Devices Regulation (MDR) 2017/745 - Clinical investigations
Regulatory requirement and approval procedure for medical devices

Regulatory requirement and approval procedure for medical devices
7 Steps - How to Get a CE Marking Certification for Medical Devices?

7 Steps - How to Get a CE Marking Certification for Medical Devices?
Similar to India medical device regulatory process
Medical Device Registration in India 

Rules and regulations for medical device registration, forms and fees required, types of medical devices to be registered under CDSCO India.
Indian market overview
India’s medical device regulatory structure
Categories of regulated medical devices in India
No Objection Certificates
Device Registration Certificate applications
Import Licensing
India Authorized Agent selection
Costs and Timelines
Developing Regulatory Issues in India
Shruthi seminar final ppt (1)

Medical device and Patency
By MDD 93/42
Any devices including an instrument, apparatus, appliance, implant, material or other article, whether used alone or in combination, including a software or an accessory, intended by its manufacturer to be used specially for human beings or animals which does not achieve the primary intended action in or on human body or animals by any pharmacological or immunological or metabolic means, but which may assist in its intended function by such means for one or more of the specific purposes of ―
diagnosis, prevention, monitoring, treatment or alleviation of any disease or disorder;
diagnosis, monitoring, treatment, alleviation or assistance for, any injury or disability;
investigation, replacement or modification or support of the anatomy or of a physiological process;
supporting or sustaining life;
disinfection of medical devices; and
control of conception.
What is 510(k) ?
A 510(k) is a premarket submission made to FDA to demonstrate
that the device to be marketed is as safe and effective, that is,
substantially equivalent, to a legally marketed device
(section 513(i)(1)(a) FD&C act).
Medical Devices are notified as DRUGS under Drugs & Cosmetics Act. Section 3 (b) (iv) defines,
Medical Devices as “Devices intended for internal or external use in the diagnosis, treatment, mitigation or prevention of disease or disorder in human beings or animals”
India had no regulation for medical devices in place prior to 2005.
The import, manufacturing, distribution and Sale of medical devices in India are overseen by the Drugs and Cosmetics Act (1940) and Rules (1945)
Control and inspection are carried out by the CDSCO, state drug controllers and central/state laboratories.
Mdr 17 with 2020 rules

Recently Govt of Indian Ministry of Health has published New Medical Device Definition According to which all medical devices are regulated
rules, regulation and guideline for medical devices

this guideline gives information about rules, regulation for medical devices.
Regulatory mechanism in india

The Central Drugs Standard Control Organization (CDSCO) is the key medical regulatory organization in India and has established a Medical Device Division to oversee matters related to notified medical devices. The Central Licensing Approval Authority (CLAA) serves as the main regulatory body for medical devices. All medical devices must undergo conformity assessment to ensure safety and quality standards are met. Obtaining registration for a medical device in India requires appointing a local representative, compiling a device application, undergoing CDSCO review which may include queries and a technical presentation, and can take 6-9 months or up to 18 months if clinical trials are required. Registration is valid for 3 years and must be renewed in advance of expiry. An import license from the CDSCO
Device Registration

The document discusses medical device regulations in India. The Drugs Controller General of India oversees the Central Drugs Standard Control Organisation, which regulates medical devices. Only notified devices are regulated, which include items like syringes, stents, and implants. Device registration requires submitting legal, regulatory, technical, and quality documents and can take 4-5 months. Import licenses for registered devices may take an additional month. The contact details of Accredited Consultants Pvt. Ltd. are provided.
Device Registration in India

The document discusses medical device regulations in India. The Drugs Controller General of India oversees the Central Drugs Standard Control Organisation, which regulates medical devices. Only notified devices are regulated, which include items like syringes, stents, and implants. Device registration requires submitting legal, regulatory, technical, and quality documents and can take 4-5 months. Import licenses for registered devices may take an additional month. The contact details of Accredited Consultants Pvt. Ltd. are provided.
Strategies for Device Approval in China, India, South Korea and Australia

This session will describe the orthopaedic device regulatory and registration requirements in Asia Pacific markets. Regulatory steps and strategies will be presented for each of these countries. The discussion will also cover ways to gain regulatory information about competitors already selling in these markets. Attendees will leave the session with an understanding of timelines, costs and complexity for approval.
Pakistan Medical Device regulatory process

Medical devices in Pakistan are regulated by the Medical Device & Medicated Cosmetics Division and must be classified according to the Medical Device Rules of 2017. The regulatory process involves appointing a registered importer, determining the classification, and having an authorized representative submit an application dossier and fees to the Medical Devices Board for approval. Approval times range from less than two months for Class A local manufacturer devices to 8-10 months for Class D imported devices not from reference countries.
Regulatory Framework: SOPs For Ethical Regulation Of Drugs

The document outlines standard operating procedures and regulatory requirements for clinical trials in India. It discusses maintaining proper documentation for regulatory compliance, including investigator files, informed consent forms, and safety reporting. The order of documents in investigator files is provided. It also summarizes the role and functions of the Central Drugs Standard Control Organisation as the approving authority for drugs, medical devices, and clinical trials in India.
Standard Operating Procedures

This document outlines standard operating procedures for regulatory affairs in the pharmaceutical industry in India. It discusses maintaining proper documentation for clinical trials, including investigator files, informed consent forms, and regulatory forms like the FDA Form 1572. It provides the order that documents should be filed and stored. It also describes the regulatory approval process and requirements for medical devices in India through the Central Drugs Standard Control Organisation.
New drug approval

The document discusses India's drug regulatory system. The Drug Controller General of India regulates drugs and medical devices in the country to ensure quality, safety and efficacy. New drugs require approval through a New Drug Application process which involves submitting documentation on manufacturing, non-clinical studies, and clinical trials for review. It takes about a year to review an NDA and various forms and fees are involved in the approval and import license application processes.
Device registration ppt

To import Drug into India, the Site of manufacturing & products are to be registered.We give Services in getting manufacturing licences
ACCREDITED CONSULTANTS PVT.LTD
info@acplgroupindia.co.in
+919310040434
Procedure for getting the manufacturing license of notified IVDs Products in ...

1) The document outlines the procedure for obtaining a manufacturing license for notified in vitro diagnostic (IVD) products in India. It involves submitting an application with documents to the state and central drug authorities, who may request additional information.
2) If approved, joint inspections are conducted by state and central inspectors. If deficiencies are found, corrections must be made and reinspection may occur. Test batches are produced and evaluated.
3) Upon receiving positive evaluation reports and recommendations, the state authority can issue the manufacturing license, which is valid for 5 years. The process generally takes 6-9 months for notified IVDs. Non-notified IVD licenses have fewer requirements.
Medical device regulations

In the USA, medical devices are regulated by the Food and Drug Administration (FDA) with an aim to ensure safety and effectiveness of the devices. The Center for Devices and Radiological Health (CDRH) is an FDA component and looks after this program.
medical device registration in china

The document provides an overview of medical device registration in China. It discusses the Chinese regulatory environment and key organizations like the China Food and Drug Administration (CFDA). The CFDA classifies medical devices and oversees the registration process, which involves choosing an agent, submitting documentation, conducting tests, making two submissions for review, and final administrative approval. Attention to small details and unpredictability are characteristics of the Chinese process.
Medical Device registration in china

The document provides an overview of medical device registration in China. It discusses the Chinese regulatory environment and key organizations like the China Food and Drug Administration (CFDA). The CFDA classifies medical devices and oversees the registration process, which involves choosing an agent, submitting documentation, conducting tests, making two submissions for review, and final administrative approval. Attention to small details and unpredictability are characteristics of the Chinese process.
New Drug Approval in India

The document summarizes India's drug regulation system. The Drug Controller General of India oversees the Central Drug Standard Control Organisation and regulates medical devices and drugs. New drugs must go through an approval process involving clinical trials and submissions to various departments before gaining an import license and being allowed for manufacture and sale in India. The process can take around one year and involves filing various regulatory, technical and legal documents with fees.
Cdsco ppt

The Central Drugs Standard Control Organisation (CDSCO) is India's national regulatory body for pharmaceuticals and medical devices. It is responsible for approving new drugs, medical devices, and clinical trials. CDSCO has headquarters in New Delhi and is overseen by the Drug Controller General of India. It has various zonal and sub-zonal offices that perform GMP audits, inspections, and quality control tests. CDSCO uses a multi-step process to approve clinical trials, drugs, cosmetics, and medical devices that involves submitting documents and gaining permission from the DCGI. It also has an online portal called SUGAM to streamline the application process.
Medical Devices Rules 2017 Implementation

Medical Devices Rules are now enforced for all medical devices. It is important to know about the MDR 2017 and how it affects the Manufacturers, Importers and Distributors of Medical Devices and status of implementation.
Similar to India medical device regulatory process (20)
rules, regulation and guideline for medical devices

rules, regulation and guideline for medical devices
Strategies for Device Approval in China, India, South Korea and Australia

Strategies for Device Approval in China, India, South Korea and Australia
Regulatory Framework: SOPs For Ethical Regulation Of Drugs

Regulatory Framework: SOPs For Ethical Regulation Of Drugs
Procedure for getting the manufacturing license of notified IVDs Products in ...

Procedure for getting the manufacturing license of notified IVDs Products in ...
Recently uploaded
Innovative Minds France's Most Impactful Healthcare Leaders.pdf

This edition features a handful of Innovative Minds: France's Most Impactful Healthcare Leaders that are leading us into a better future.
TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardso...

TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardso...rightmanforbloodline
TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardson, Verified Chapters 1 - 18, Complete Newest Version
TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardson, Verified Chapters 1 - 18, Complete Newest Version
TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardson, Verified Chapters 1 - 18, Complete Newest VersionLGBTQ+ Adults: Unique Opportunities and Inclusive Approaches to Care

This webinar helps clinicians understand the unique healthcare needs of the LGBTQ+ community, primarily in relation to end-of-life care. Topics include social and cultural background and challenges, healthcare disparities, advanced care planning, and strategies for reaching the community and improving quality of care.
Letter to MREC - application to conduct study

Application to conduct study on research title 'Awareness and knowledge of oral cancer and precancer among dental outpatient in Klinik Pergigian Merlimau, Melaka'
Can Allopathy and Homeopathy Be Used Together in India.pdf

This article explores the potential for combining allopathy and homeopathy in India, examining the benefits, challenges, and the emerging field of integrative medicine.
Top Rated Massage Center In Ajman Chandrima Spa

The best massage spa Ajman is Chandrima Spa Ajman, which was founded in 2023 and is exclusively for men 24 hours a day. As of right now, our parent firm has been providing massage services to over 50,000+ clients in Ajman for the past 10 years. It has about 8+ branches. This demonstrates that Chandrima Spa Ajman is among the most reasonably priced spas in Ajman and the ideal place to unwind and rejuvenate. We provide a wide range of Spa massage treatments, including Indian, Pakistani, Kerala, Malayali, and body-to-body massages. Numerous massage techniques are available, including deep tissue, Swedish, Thai, Russian, and hot stone massages. Our massage therapists produce genuinely unique treatments that generate a revitalized sense of inner serenely by fusing modern techniques, the cleanest natural substances, and traditional holistic therapists.
Gemma Wean- Nutritional solution for Artemia

GEMMA Wean is a high end larval co-feeding and weaning diet aimed at Artemia optimisation and is fortified with a high level of proteins and phospholipids. GEMMA Wean provides the early weaned juveniles with dedicated fish nutrition and is an ideal follow on from GEMMA Micro or Artemia.
GEMMA Wean has an optimised nutritional balance and physical quality so that it flows more freely and spreads readily on the water surface. The balance of phospholipid classes to- gether with the production technology based on a low temperature extrusion process improve the physical aspect of the pellets while still retaining the high phospholipid content.
GEMMA Wean is available in 0.1mm, 0.2mm and 0.3mm. There is also a 0.5mm micro-pellet, GEMMA Wean Diamond, which covers the early nursery stage from post-weaning to pre-growing.
Stem Cell Solutions: Dr. David Greene's Path to Non-Surgical Cardiac Care

Explore the groundbreaking work of Dr. David Greene, a pioneer in regenerative medicine, who is revolutionizing the field of cardiology through stem cell therapy in Arizona. This ppt delves into how Dr. Greene's innovative approach is providing non-surgical, effective treatments for heart disease, using the body's own cells to repair heart damage and improve patient outcomes. Learn about the science behind stem cell therapy, its benefits over traditional cardiac surgeries, and the promising future it holds for modern medicine. Join us as we uncover how Dr. Greene's commitment to stem cell research and therapy is setting new standards in healthcare and offering new hope to cardiac patients.
Under Pressure : Kenneth Kruk's Strategy

Kenneth Kruk's story of transforming challenges into opportunities by leading successful medical record transitions and bridging scientific knowledge gaps during COVID-19.
PrudentRx's Function in the Management of Chronic Illnesses

PrudentRx ensures effective, cost-efficient medication management for chronic diseases, enhancing patient health and productivity.
Luxurious Spa In Ajman Chandrima Massage Center

Chandrima Spa Ajman is one of the leading Massage Center in Ajman, which is open 24 hours exclusively for men. Being one of the most affordable Spa in Ajman, we offer Body to Body massage, Kerala Massage, Malayali Massage, Indian Massage, Pakistani Massage Russian massage, Thai massage, Swedish massage, Hot Stone Massage, Deep Tissue Massage, and many more. Indulge in the ultimate massage experience and book your appointment today. We are confident that you will leave our Massage spa feeling refreshed, rejuvenated, and ready to take on the world.
Visit : https://massagespaajman.com/
Call : 052 987 1315
DRAFT Ventilator Rapid Reference version 2.4.pdf

Ventilator rapid reference for the Zoll Z cvent. Presented for educational purposes only.
Champions of Health Spotlight On Leaders Shaping Germany's Healthcare.pdf

This edition features a handful of Champions of Health: Spotlight On Leaders Shaping Germany's Healthcare that are leading us into a better future.
Empowering ACOs: Leveraging Quality Management Tools for MIPS and Beyond

Join us as we delve into the crucial realm of quality reporting for MSSP (Medicare Shared Savings Program) Accountable Care Organizations (ACOs).
In this session, we will explore how a robust quality management solution can empower your organization to meet regulatory requirements and improve processes for MIPS reporting and internal quality programs. Learn how our MeasureAble application enables compliance and fosters continuous improvement.
INFECTION OF THE BRAIN -ENCEPHALITIS ( PPT)

Neurological system includes brain and spinal cord. It plays an important role in functioning of our body. Encephalitis is the inflammation of the brain. Causes include viral infections, infections from insect bites or an autoimmune reaction that affects the brain. It can be life-threatening or cause long-term complications. Treatment varies, but most people require hospitalization so they can receive intensive treatment, including life support.
Pediatric Emergency Care for Children | Apollo Hospital

Pediatric Emergency Care for Children | Apollo HospitalApollo 24/7 Adult & Paediatric Emergency Services
At Apollo Hospital, Lucknow, U.P., we provide specialized care for children experiencing dehydration and other symptoms. We also offer NICU & PICU Ambulance Facility Services. Consult our expert today for the best pediatric emergency care.
For More Details:
Map: https://cutt.ly/BwCeflYo
Name: Apollo Hospital
Address: Singar Nagar, LDA Colony, Lucknow, Uttar Pradesh 226012
Phone: 08429021957
Opening Hours: 24X7Deep Leg Vein Thrombosis (DVT): Meaning, Causes, Symptoms, Treatment, and Mor...

Deep Leg Vein Thrombosis (DVT): Meaning, Causes, Symptoms, Treatment, and Mor...The Lifesciences Magazine
Deep Leg Vein Thrombosis occurs when a blood clot forms in one or more of the deep veins in the legs. These clots can impede blood flow, leading to severe complications.RECENT ADVANCES IN BREAST CANCER RADIOTHERAPY

ULTRA HYPOFRACTIONATION
Accelerated Partial Breast Irradiation: :Brachytherapy
UK FAST FORWARD
Can coffee help me lose weight? Yes, 25,422 users in the USA use it for that ...

The South Beach Coffee Java Diet is a variation of the popular South Beach Diet, which was developed by cardiologist Dr. Arthur Agatston. The original South Beach Diet focuses on consuming lean proteins, healthy fats, and low-glycemic index carbohydrates. The South Beach Coffee Java Diet adds the element of coffee, specifically caffeine, to enhance weight loss and improve energy levels.
Recently uploaded (20)
Innovative Minds France's Most Impactful Healthcare Leaders.pdf

Innovative Minds France's Most Impactful Healthcare Leaders.pdf
TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardso...

TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardso...
LGBTQ+ Adults: Unique Opportunities and Inclusive Approaches to Care

LGBTQ+ Adults: Unique Opportunities and Inclusive Approaches to Care
Can Allopathy and Homeopathy Be Used Together in India.pdf

Can Allopathy and Homeopathy Be Used Together in India.pdf
Stem Cell Solutions: Dr. David Greene's Path to Non-Surgical Cardiac Care

Stem Cell Solutions: Dr. David Greene's Path to Non-Surgical Cardiac Care
PrudentRx's Function in the Management of Chronic Illnesses

PrudentRx's Function in the Management of Chronic Illnesses
Champions of Health Spotlight On Leaders Shaping Germany's Healthcare.pdf

Champions of Health Spotlight On Leaders Shaping Germany's Healthcare.pdf
Empowering ACOs: Leveraging Quality Management Tools for MIPS and Beyond

Empowering ACOs: Leveraging Quality Management Tools for MIPS and Beyond
Pediatric Emergency Care for Children | Apollo Hospital

Pediatric Emergency Care for Children | Apollo Hospital
Deep Leg Vein Thrombosis (DVT): Meaning, Causes, Symptoms, Treatment, and Mor...

Deep Leg Vein Thrombosis (DVT): Meaning, Causes, Symptoms, Treatment, and Mor...
Can coffee help me lose weight? Yes, 25,422 users in the USA use it for that ...

Can coffee help me lose weight? Yes, 25,422 users in the USA use it for that ...
India medical device regulatory process
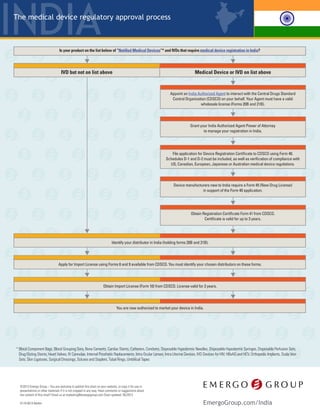
- 1. India © 2016 Emergo – Have comments or suggestions about the content of this chart? Email us at marketing@emergogroup.com. Chart updated 09/2016. EmergoGroup.com/India Medical devices and IVDs are regulated by the Drug Controller General of India (DCGI) within the Central Drugs Standard Control Organization (CDSCO); part of the Ministry of Health and Family Welfare. The regulatory framework for medical devices is based on drug regulations under the Drugs and Cosmetics Act of 1940, and Drugs and Cosmetics Rules of 1945. • Ablation Devices† • Blood Component Bags • Blood Grouping Sera • Bone Cements • Cardiac Stents • Catheters • Condoms • Dental Implants† • Disposable Hypodermic Needles • Disposable Hypodermic Syringes • Disposable Perfusion Sets • Drug Eluting Stents • Heart Valves • HBsAG and HCV • IV Cannulae • IVD Devices for HIV • Internal Prosthetic Replacements • Intra Ocular Lenses • Intra Uterine Devices • Orthopedic Implants • Scalp Vein Sets • Skin Ligatures • Surgical Dressings • Sutures and Staplers • Tubal Rings • Umbilical Tapes Currently only a limited number of medical devices and IVDs require registration in India.† They are: Appoint an India Authorized Agent to interact with the CDSCO on your behalf. Your Agent must have a valid wholesale license (Forms 20B and 21B/21C), and be granted Power of Attorney to manage your registration in India. Compile device application (Form 40), including: manufacturing facility information, device technical information, ISO 13485 certificate, IFU, testing results (if applicable), clinical data (if applicable), proof of approval in: the US, EU, Australia, Canada, or Japan, plus proof of approval in your home country (satisfied by CFS/CFG). File registration applications with the CDSCO. Pay fees. All documents must be in English. The CDSCO will issue a Registration Certificate (Form 41), which is valid for 3 years. The CDSCO reviews applications and may require a Technical Presentation.** Novel devices will also undergo a Subject Expert Committee (SEC) review.*** Notified IVDs require in-country performance testing through the National Institute of Biologicals (NIB). Seven types of non-notified IVDs and certain products for testing blood glucose require in-country performance testing. Results will be submitted with your import license application.* Non-notified IVD (NOT on list above) Notified Medical Device or IVD (On list above) Identify your distributor in India and apply for Import License. Qualified distributors must hold Forms 20B and 21B/21C. Obtain Import License (Form 10) from the CDSCO. The Import License will be issued in the name of your distributor. You are now authorized to market your device or IVD in India. 5119-0916 Regulatory Process for Medical Devices and IVDs † Some products that do not appear on the official list still require registration. Ablation devices, dental implants, and hernia mesh are examples. All IVDs, including Notified and Non-Notified, require an Import License. Medical devices which do not require registration have no restrictions and may be imported directly. Please contact us for details. * The seven types of non-notified IVDs requiring performance testing are: malaria, dengue, chikungunya, syphilis, typhoid, tuberculosis, and cancer markers. Testing must be conducted by a local accredited test laboratory. Blood glucose test strips and fully automated analyser based glucose reagents require performance testing from NIB (NIB also tests Notified IVDs). ** Approximately 25% of applications require a formal Technical Presentation. The Technical Presentation is an in-person meeting with the CDSCO to discuss the product in more detail. A representative from the manufacturer (such as an engineer) is expected to attend this meeting along with the India Authorized Agent. *** Devices novel to the Indian market (new technology, material, intended use), may face additional regulatory hurdles. CDSCO may require clinical studies conducted in India prior to regulatory approval, or the agency may issue a restricted approval. A restricted approval could include a requirement to actively collect and submit post-market data. The SEC meeting will include local clinicians and other experts who will weigh in on the acceptability of the existing clinical data. This is a simplified overview of the process. The CDSCO may choose to audit your submission and request more documents, which will add time to your approval.
- 2. India Notes 1. The time frames shown above are typical for the majority of medical device submissions but assume that your device does not contain animal tissue, medicinal substances or employ entirely novel technology. Your length of approval will depend on the quality and completeness of your technical documentation and how much time you take to address additional information requests from authorities after submission. YOUR SUBMISSION(S) MAY TAKE MORE TIME THAN WHAT IS SHOWN ABOVE. 2. Registrations remain valid for the time specified as long as you do not make changes to the device, intended use or indications for use. 3. We recommend starting the re-registration process no later than the time period specified above. However, please consult with your distributor or regulatory expert well before this suggested time to avoid any lapse in your registration. 4. Our rating of the complexity of the registration process is based on our experience and the opinion of nearly 1,000 QA/RA professionals worldwide who were asked to rate the difficulty of registering a device in each country. The European CE Marking process is considered the mid-point to which all other markets are compared. 5. Low = Less than US$5000; Midpoint = US$15000-$30000: High = More than US$50000. Overall cost includes registration application fees, product testing, in-country representation, submission preparation consulting and translation of registration documents but not IFU. Costs assume you already have approval for your device in the United States, Europe, Canada or Japan. Does not include cost of implementing or auditing a quality management system compliant with ISO 13485 or US FDA 21 CFR Part 820, if applicable. EmergoGroup.com/India Device classification in India Non-notified IVD Notified Device or IVD How long you should expect to wait after submission until approval is granted.1 < 1 month 9-12 months* Validity period for device registrations.2 3 years** 3 years Renewal should be started this far in advance.3 3 months** 1 year Complexity of the registration process.4 Overall cost of gaining regulatory approval.5 5119-0916 © 2016 Emergo – Have comments or suggestions about the content of this chart? Email us at marketing@emergogroup.com. Chart updated 09/2016. Time, Cost, and Complexity of Registration Simple ComplexSimple Complex Low HighLow High * If required, a Technical Presentation or Subject Expert Committee (SEC) Meeting will increase the approval timeline by three to six months. ** Non-notified IVDs do not require registration but do require an import license. This license is valid for three years. This is a simplified overview of the process. The CDSCO may choose to audit your submission and request more documents, which will add time to your approval.
