Europe IVD medical registration and approval chart - EMERGO
•
2 likes•4,747 views
The document summarizes the regulatory process for in vitro diagnostic devices (IVDs) in Europe under the In Vitro Diagnostic Directive (98/79/EC). It outlines the classification of IVDs, requirements for quality management systems and technical files, roles of notified bodies and authorized representatives, and timelines and costs associated with the approval process depending on the IVD classification. The process can take from 3-5 months for self-certified IVDs to 9-12 months for list A IVDs and involves implementing quality systems, obtaining notified body audits, and registering with authorities.
Report
Share
Report
Share
Download to read offline
Recommended
Europe CE Marking for medical devices under new MDR

This chart explains the new European Medical Device Regulation registration process under the new MDR.
Brazil medical device registration and approval chart - EMERGO

Easy to understand chart describes the ANVISA medical device registration process in Brazil.
Japan medical device approval chart - Emergo 

Easy to understand chart describes the PMDA medical device registration process in Japan.
Regulation of medical device in japan

Medical Devices registration in Japan , quality system requirements and evaluation and investigation of medical devices in regulated countries ASEAN, China JAPAN and WHO regulations. quality and ethical considerations regulatory and documentation requirements for marketing medical devices and IVDs in regulated countries.
Regulatory Approval Process for Medical Devices in EU - Presentation by Aksha...

A presentation on Regulatory Approval Process for Medical Devices in European Union that explains in brief about the various aspects including the EU Medical Device Directives, Classifications, CE Certification, Medical Device Registration & Timelines. This was presented as a part of curriculum by Akshay Anand in JSS College of Pharmacy, Mysuru during January 2015
US FDA medical device approval chart - Emergo 

Simple one page chart shows the US FDA medical device approval process for Class 1, 2 and 3 devices. Very easy to understand.
Recommended
Europe CE Marking for medical devices under new MDR

This chart explains the new European Medical Device Regulation registration process under the new MDR.
Brazil medical device registration and approval chart - EMERGO

Easy to understand chart describes the ANVISA medical device registration process in Brazil.
Japan medical device approval chart - Emergo 

Easy to understand chart describes the PMDA medical device registration process in Japan.
Regulation of medical device in japan

Medical Devices registration in Japan , quality system requirements and evaluation and investigation of medical devices in regulated countries ASEAN, China JAPAN and WHO regulations. quality and ethical considerations regulatory and documentation requirements for marketing medical devices and IVDs in regulated countries.
Regulatory Approval Process for Medical Devices in EU - Presentation by Aksha...

A presentation on Regulatory Approval Process for Medical Devices in European Union that explains in brief about the various aspects including the EU Medical Device Directives, Classifications, CE Certification, Medical Device Registration & Timelines. This was presented as a part of curriculum by Akshay Anand in JSS College of Pharmacy, Mysuru during January 2015
US FDA medical device approval chart - Emergo 

Simple one page chart shows the US FDA medical device approval process for Class 1, 2 and 3 devices. Very easy to understand.
regulatory aspects of medical devices in USA

introduction, classification, regulatory approval process for medical devices (510k) premarket notification, pre market approval (PMA), investigational device exemption (IDE) and invitro diagnostics, quality system requirements 21 CFR PART 820, labeling requirements 21 CFR part 801, UDI
China: Medical Device Regulations

This whitepaper provides an overview of Chinese Medical Device Regulations. This includes an overview of the Chinese medical device market, medical device regulatory authorities, medical device registration procedure and medical device classification. It also provides information on regulations regarding product standard, type testing, and clinical trials. This paper is meant for anyone within the regulatory affairs industry who is looking to learn more about medical device regulations and product registration in China.
For more information, contact us for a free 15 minute consultation at http://www.pacificbridgemedical.com/contact-us/.
Regulatory requirements for CE CERTIFICATION of Medical Devices in European U...

The EU introduced the CE marking scheme to make trade easier and cheaper between EU countries. It means that a manufacturer claims that their product conforms to the minimum legal requirements for health and safety as laid down in EU directives. In addition, it may be considered a benefit that by implementing the requirements, the product will be safer for the user and this may also reduce damage and liability claims. Additional benefits may include your product being made safer for end-users. The EU introduced the CE marking scheme to make trade easier and cheaper between EU countries. It means that a manufacturer claims that their product conforms to the minimum legal requirements for health and safety as laid down in EU directives. If you manufacture or import a product which falls within the scope of one or more of the New Approach Directives and wish to place your product on the market in any of the member’s states of the European Economic Area (EEA), then you must apply CE marking to your product against the essential requirements of all these applicable directives.
Key Words: European Union, CE marking, New Approach Directives, EEA, Regulatory requirements.
MARKETING AUTHORISATION, LICENSING AND QUALITY ASSESSMENT OF VACCINES IN INDI...

- European pharmaceutical legislation provides a comprehensive framework for the marketing authorisation of vaccines.
- In contrast to the European scenario, the Indian scenario for vaccines is relatively less regulated and follows the same process of approval as other biologics in spite of having a National Handbook for Vaccine Policy.
- Vaccine authorisation in the US, as is the case in EU, is a more straightforward process than in most other markets as the USFDA has provided vaccines with a distinct set of regulations in concerned areas of safety and quality.
The In vitro diagnostic medical devices regulation (EU) 2017/746: what will c...

The In vitro diagnostic medical devices regulation (EU) 2017/746: what will c...European Center for Disease Prevention and Control (ECDC)
Presentation of the 18th ECDC National Focal Points for Microbiology (NMFP) meeting held on 3-4 May 2018 in StockholmThe European Medical Device Regulations - analysis of the final text

Following the publication of the new European Medical Device Regulations by the European Commission, we offer you our analysis of the final text.
Colombia medical device approval chart - Emergo 

Easy to understand chart describes the medical device registration process with INVIMA in Colombia.
Canada medical device approval chart - EMERGO

Easy to understand chart describes the medical device registration process with Health Canada.
Medical Devices Regulation (MDR) 2017/745 - Clinical investigations

Medical Devices Regulation (MDR) 2017/745 - Clinical investigations
See more https://www.aretezoe.com/medical-devices
TSE/BSE Evaluation

TSE/BSE is a type of disease affected to the animals which may transmit to the humans if any products obtained by the disease caused animal may affect to humans also
The many biologic products are expracted from the animal source so before the extraction the animal should be tested for TSE/BSE organism in their source/Body
FDA Unique Device Identification (UDI) Overview

Presentation on UDI for Grace College Orthopaedic Regulatory and Clinical Affairs graduate program (RCA5700)
plasma master file in European countries and requirements in letter of intent...

it includes that what is plasma master file, principles, procedure to file PMF, strategy involved, administration information, certification procedure & inspection
Medical device approval chart for Russia - EMERGO

Easy to understand chart describes the medical device registration process in Russia.
China medical device approval chart - EMERGO

Easy to understand chart describes the CFDA medical device registration process in China.
More Related Content
What's hot
regulatory aspects of medical devices in USA

introduction, classification, regulatory approval process for medical devices (510k) premarket notification, pre market approval (PMA), investigational device exemption (IDE) and invitro diagnostics, quality system requirements 21 CFR PART 820, labeling requirements 21 CFR part 801, UDI
China: Medical Device Regulations

This whitepaper provides an overview of Chinese Medical Device Regulations. This includes an overview of the Chinese medical device market, medical device regulatory authorities, medical device registration procedure and medical device classification. It also provides information on regulations regarding product standard, type testing, and clinical trials. This paper is meant for anyone within the regulatory affairs industry who is looking to learn more about medical device regulations and product registration in China.
For more information, contact us for a free 15 minute consultation at http://www.pacificbridgemedical.com/contact-us/.
Regulatory requirements for CE CERTIFICATION of Medical Devices in European U...

The EU introduced the CE marking scheme to make trade easier and cheaper between EU countries. It means that a manufacturer claims that their product conforms to the minimum legal requirements for health and safety as laid down in EU directives. In addition, it may be considered a benefit that by implementing the requirements, the product will be safer for the user and this may also reduce damage and liability claims. Additional benefits may include your product being made safer for end-users. The EU introduced the CE marking scheme to make trade easier and cheaper between EU countries. It means that a manufacturer claims that their product conforms to the minimum legal requirements for health and safety as laid down in EU directives. If you manufacture or import a product which falls within the scope of one or more of the New Approach Directives and wish to place your product on the market in any of the member’s states of the European Economic Area (EEA), then you must apply CE marking to your product against the essential requirements of all these applicable directives.
Key Words: European Union, CE marking, New Approach Directives, EEA, Regulatory requirements.
MARKETING AUTHORISATION, LICENSING AND QUALITY ASSESSMENT OF VACCINES IN INDI...

- European pharmaceutical legislation provides a comprehensive framework for the marketing authorisation of vaccines.
- In contrast to the European scenario, the Indian scenario for vaccines is relatively less regulated and follows the same process of approval as other biologics in spite of having a National Handbook for Vaccine Policy.
- Vaccine authorisation in the US, as is the case in EU, is a more straightforward process than in most other markets as the USFDA has provided vaccines with a distinct set of regulations in concerned areas of safety and quality.
The In vitro diagnostic medical devices regulation (EU) 2017/746: what will c...

The In vitro diagnostic medical devices regulation (EU) 2017/746: what will c...European Center for Disease Prevention and Control (ECDC)
Presentation of the 18th ECDC National Focal Points for Microbiology (NMFP) meeting held on 3-4 May 2018 in StockholmThe European Medical Device Regulations - analysis of the final text

Following the publication of the new European Medical Device Regulations by the European Commission, we offer you our analysis of the final text.
Colombia medical device approval chart - Emergo 

Easy to understand chart describes the medical device registration process with INVIMA in Colombia.
Canada medical device approval chart - EMERGO

Easy to understand chart describes the medical device registration process with Health Canada.
Medical Devices Regulation (MDR) 2017/745 - Clinical investigations

Medical Devices Regulation (MDR) 2017/745 - Clinical investigations
See more https://www.aretezoe.com/medical-devices
TSE/BSE Evaluation

TSE/BSE is a type of disease affected to the animals which may transmit to the humans if any products obtained by the disease caused animal may affect to humans also
The many biologic products are expracted from the animal source so before the extraction the animal should be tested for TSE/BSE organism in their source/Body
FDA Unique Device Identification (UDI) Overview

Presentation on UDI for Grace College Orthopaedic Regulatory and Clinical Affairs graduate program (RCA5700)
plasma master file in European countries and requirements in letter of intent...

it includes that what is plasma master file, principles, procedure to file PMF, strategy involved, administration information, certification procedure & inspection
What's hot (20)
Regulatory requirements for CE CERTIFICATION of Medical Devices in European U...

Regulatory requirements for CE CERTIFICATION of Medical Devices in European U...
MARKETING AUTHORISATION, LICENSING AND QUALITY ASSESSMENT OF VACCINES IN INDI...

MARKETING AUTHORISATION, LICENSING AND QUALITY ASSESSMENT OF VACCINES IN INDI...
The In vitro diagnostic medical devices regulation (EU) 2017/746: what will c...

The In vitro diagnostic medical devices regulation (EU) 2017/746: what will c...
The European Medical Device Regulations - analysis of the final text

The European Medical Device Regulations - analysis of the final text
Regulatory approval process for invitro diagnostics in us

Regulatory approval process for invitro diagnostics in us
Medical Devices Regulation (MDR) 2017/745 - Clinical investigations

Medical Devices Regulation (MDR) 2017/745 - Clinical investigations
Medical device regulation US, European Union and India

Medical device regulation US, European Union and India
Market authorisation checklist for brics countries

Market authorisation checklist for brics countries
plasma master file in European countries and requirements in letter of intent...

plasma master file in European countries and requirements in letter of intent...
Viewers also liked
Medical device approval chart for Russia - EMERGO

Easy to understand chart describes the medical device registration process in Russia.
China medical device approval chart - EMERGO

Easy to understand chart describes the CFDA medical device registration process in China.
India medical device regulatory process

Easy to understand chart describes the medical device registration process with CDSCO in India.
Japan PMDA Medical Device Regulatory Approval Process

Watch the recorded webinar: https://www.emergogroup.com/resources/video-webinar-japan-registration-process
Japan's medical device market is one of the most robust markets in Asia, but its regulatory system can be confusing. Before deciding to sell your device in the Japanese market, it is important to understand how the regulations apply to your device, which steps to take, and what resources are required to complete the process. In this presentation, Ann Marie Boullie, Vice President of Business Development for EMERGO, will discuss some of the most complex aspects of the Japanese registration process, including:
JMDN codes: device classification and predicates
Clinical data requirements and PMDA pre-submission meetings
Registration routes (Todokede, Ninsho, Shonin)
QMS (Ordinance 169) requirements
Role of the Marketing Authorization Holder (MAH)
Plus more ...
Leveraging Oracle IDMP Enterprise Foundation Suite for Regulatory Compliance

IDMP (Identification of Medicinal Products), which will soon be mandated by the European Medicines Agency (EMA) and U.S. Food and Drug Administration (FDA), will enable stakeholders to obtain a comprehensive view of each individual product (e.g., ingredients, marketing and medicinal information, contacts), based on unique codes.
While the journey towards IDMP compliance can be incredibly challenging, industry-specific knowledge and systems play an integral role in meeting the new requirements.
In our webinar, we discussed how the Oracle IDMP Enterprise Foundation Suite can help you be ready in time.
UL Webinar - Updates to the Regulatory Framework in Korea and their impact to...

fIn this presentation UL technical expert, MinYong Choi, formerly with the Korea FDA provides a comprehensive overview of the Regulatory framework for medical and IVD devices in Korea. The presentation includes useful links, and insight into recent and planned changes to the regulations that may affect submissions for market approval.
Australia medical device registration and approval process - EMERGO

Easy to understand chart describes the medical device registration process in Australia.
Medical Device registration in china

This short presentation is giving a brief introduction of the general status of china medial device industry, as well as an explanation about the CFDA and the medical registration process in china
From documents to datasets and back: challenges and solutions 

The upcoming effectuation of the IDMP directive (EU) forces pharma companies to submit datasets instead of documents. Based on state of the art entity extraction software, a solution is developed that generates those parts of the dataset that can be obtained from the text. The presentation describes some of the major challenges that had to be overcome and details the solutions that were found. It presents some results and describes the major business requirements that need to be met. Recognizing named entities, such as headache and nausea, in the text is not enough. The extraction software needs to be embedded in a layer of software that analyses the text and recognizes which entities are the value of which properties.
Medical device approval chart for Mexico - Emergo 

Easy to understand chart describes the medical device registration process in Mexico for COFEPRIS.
South Korea medical device approval chart - Emergo 

Easy to understand chart describes the KFDA medical device registration process in South Korea.
Taiwan medical device registration and approval chart - EMERGO

Easy to understand chart describes the medical device registration process with the TFDA in Taiwan.
Viewers also liked (16)
Japan PMDA Medical Device Regulatory Approval Process

Japan PMDA Medical Device Regulatory Approval Process
Leveraging Oracle IDMP Enterprise Foundation Suite for Regulatory Compliance

Leveraging Oracle IDMP Enterprise Foundation Suite for Regulatory Compliance
UL Webinar - Updates to the Regulatory Framework in Korea and their impact to...

UL Webinar - Updates to the Regulatory Framework in Korea and their impact to...
Australia medical device registration and approval process - EMERGO

Australia medical device registration and approval process - EMERGO
From documents to datasets and back: challenges and solutions 

From documents to datasets and back: challenges and solutions
South Korea medical device approval chart - Emergo 

South Korea medical device approval chart - Emergo
Taiwan medical device registration and approval chart - EMERGO

Taiwan medical device registration and approval chart - EMERGO
Choosing the right facility for clinical trials in Russia

Choosing the right facility for clinical trials in Russia
Similar to Europe IVD medical registration and approval chart - EMERGO
IVDR Readiness Checklist

In May 2022, the European In Vitro Diagnostics Regulation (IVDR) will apply in the world’s second-largest medical device market. The new Regulation will introduce major changes to how manufacturers obtain CE Marking and maintain access to the European market. Many companies have yet to prepare for compliance to these new requirements or organize their regulatory transition strategies. Oliver will present the ‘What will it take? Review IVDR readiness” to help you understand the scope of the new regulations.
This session took place live at the Greenlight Guru True Quality Virtual Summit, a three-day event for medical device professionals to learn to get their devices to market faster, stay ahead of regulatory changes, and use quality as their multiplier to grow their device business.
CE Mark: Where to Start

Who needs a CE mark and how do I get one? It is EU law that every product that enters the European Union meet the CE Directives and applicable Standards. This mark can only be applied to your product when you have fully complied with all relevant Directives and Standards for the type of system you are marketing and and a Declaration of Conformity has been developed.
PECB Webinar: Proposed changes for medical device quality management systems ...

We will cover:
• Overview of proposed changes to ISO 13485:201X, MDSAP
• New EU regulations and unannounced audits
• New directions for QMS and regulatory audits
Presenter:
This webinar will be presented by Danny Kroo, the founder and principal consultant at Docusys Corporation.
BER Documentary Evidence – Certification

In this webinar on Certification we are going to explain the various sources of certified data available to Assessors.
Most of these resources have been available since the introduction of BER certification in 2007. While some more experienced Assessors may feel some of the topics should be well known at this point, the purpose of the presentation is to accommodate Assessors of all levels of experience and knowledge.
It is hoped that this Webinar will help Assessors’ understanding of the evidence requirements and acceptable sources of certified data to support DEAP inputs.
From UE to the World

The IEC CB (Certified Body) scheme is the largest international product certification scheme.
The scheme is the world’s first truly international system for mutual acceptance of test reports and certificates dealing with safety of electrical and electronic components, equipment and products.
A CB-certificate is documentation of product compliance with the relevant standard. A certificate gives access to, and simplifies national certification, in more than 60 member nations and is also widely recognized in other countries.
7 Steps - How to Get a CE Marking Certification for Medical Devices?

7 Steps - How to Get a CE Marking Certification for Medical Devices?
Medical devices: 7 steps to CE-mark, and post-market surveillance

The 7 steps medical device manufacturers have to take to fulfill the requirements of EU-MDR (medical medical device regulation) and to obtain the CE mark. The quick start guide.
Cost records and audit rules 2014

New cost audirt and record rules 2014 under section 148 of Companies Act, 2013
Cost records and audit rules 2014

New Cost Audit and Record Rules under section 148 of Companies Act, 2013
Newsletter on PPE: Classification of PPE and CE Certification Process in EU

In the wake of the high demand for PPE and medical devices in the EU, Chinese suppliers have restarted exporting to Europe. However, the European Safety Federation has identified some suspicious CE certificates for both PPE and medical devices. The suppliers have either used fake documents or showed certificates by the wrong authorities. Since a CE mark is compulsory for all PPE and medical devices exported to Europe, this newsletter delves into product classification for PPE and medical devices and product classification by EU regulation. There are 2 regulations, namely, Regulation (EU) 2016/425, which is on PPE and Regulation (EU) 2017/745, which is on medical devices and accessories. It has been mandated that all medical products and PPE to be sold in the EU need to come under any of these regulations. All suppliers should have knowledge about the procedure for Certified CE Mark, the risk categories, classification of medical devices and types of notified bodies as well as their names in the regulations.
Similar to Europe IVD medical registration and approval chart - EMERGO (20)
CE Marking , FDA Approval and Associated Regulations for Wellness

CE Marking , FDA Approval and Associated Regulations for Wellness
PECB Webinar: Proposed changes for medical device quality management systems ...

PECB Webinar: Proposed changes for medical device quality management systems ...
7 Steps - How to Get a CE Marking Certification for Medical Devices?

7 Steps - How to Get a CE Marking Certification for Medical Devices?
Medical devices: 7 steps to CE-mark, and post-market surveillance

Medical devices: 7 steps to CE-mark, and post-market surveillance
Newsletter on PPE: Classification of PPE and CE Certification Process in EU

Newsletter on PPE: Classification of PPE and CE Certification Process in EU
PECB Webinar: The challenges of medical devices and laboratory quality manage...

PECB Webinar: The challenges of medical devices and laboratory quality manage...
Recently uploaded
VERIFICATION AND VALIDATION TOOLKIT Determining Performance Characteristics o...

VERIFICATION AND VALIDATION TOOLKIT Determining Performance Characteristics of Qualitative Assays
Preventing Pickleball Injuries & Treatment

We understand the unique challenges pickleball players face and are committed to helping you stay healthy and active. In this presentation, we’ll explore the three most common pickleball injuries and provide strategies for prevention and treatment.
Secret Tantric VIP Erotic Massage London

Welcome to Secret Tantric, London’s finest VIP Massage agency. Since we first opened our doors, we have provided the ultimate erotic massage experience to innumerable clients, each one searching for the very best sensual massage in London. We come by this reputation honestly with a dynamic team of the city’s most beautiful masseuses.
India Clinical Trials Market: Industry Size and Growth Trends [2030] Analyzed...![India Clinical Trials Market: Industry Size and Growth Trends [2030] Analyzed...](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![India Clinical Trials Market: Industry Size and Growth Trends [2030] Analyzed...](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
According to TechSci Research report, "India Clinical Trials Market- By Region, Competition, Forecast & Opportunities, 2030F," the India Clinical Trials Market was valued at USD 2.05 billion in 2024 and is projected to grow at a compound annual growth rate (CAGR) of 8.64% through 2030. The market is driven by a variety of factors, making India an attractive destination for pharmaceutical companies and researchers. India's vast and diverse patient population, cost-effective operational environment, and a large pool of skilled medical professionals contribute significantly to the market's growth. Additionally, increasing government support in streamlining regulations and the growing prevalence of lifestyle diseases further propel the clinical trials market.
Growing Prevalence of Lifestyle Diseases
The rising incidence of lifestyle diseases such as diabetes, cardiovascular diseases, and cancer is a major trend driving the clinical trials market in India. These conditions necessitate the development and testing of new treatment methods, creating a robust demand for clinical trials. The increasing burden of these diseases highlights the need for innovative therapies and underscores the importance of India as a key player in global clinical research.
CHAPTER 1 SEMESTER V - ROLE OF PEADIATRIC NURSE.pdf

Pediatric nurses play a vital role in the health and well-being of children. Their responsibilities are wide-ranging, and their objectives can be categorized into several key areas:
1. Direct Patient Care:
Objective: Provide comprehensive and compassionate care to infants, children, and adolescents in various healthcare settings (hospitals, clinics, etc.).
This includes tasks like:
Monitoring vital signs and physical condition.
Administering medications and treatments.
Performing procedures as directed by doctors.
Assisting with daily living activities (bathing, feeding).
Providing emotional support and pain management.
2. Health Promotion and Education:
Objective: Promote healthy behaviors and educate children, families, and communities about preventive healthcare.
This includes tasks like:
Administering vaccinations.
Providing education on nutrition, hygiene, and development.
Offering breastfeeding and childbirth support.
Counseling families on safety and injury prevention.
3. Collaboration and Advocacy:
Objective: Collaborate effectively with doctors, social workers, therapists, and other healthcare professionals to ensure coordinated care for children.
Objective: Advocate for the rights and best interests of their patients, especially when children cannot speak for themselves.
This includes tasks like:
Communicating effectively with healthcare teams.
Identifying and addressing potential risks to child welfare.
Educating families about their child's condition and treatment options.
4. Professional Development and Research:
Objective: Stay up-to-date on the latest advancements in pediatric healthcare through continuing education and research.
Objective: Contribute to improving the quality of care for children by participating in research initiatives.
This includes tasks like:
Attending workshops and conferences on pediatric nursing.
Participating in clinical trials related to child health.
Implementing evidence-based practices into their daily routines.
By fulfilling these objectives, pediatric nurses play a crucial role in ensuring the optimal health and well-being of children throughout all stages of their development.
VVIP Dehradun Girls 9719300533 Heat-bake { Dehradun } Genteel ℂall Serviℂe By...

VVIP Dehradun Girls 9719300533 Heat-bake { Dehradun } Genteel ℂall Serviℂe By Our Agency
Navigating Women's Health: Understanding Prenatal Care and Beyond

Navigating Women's Health with Aboud Health Group. Explore comprehensive care options for women's wellness needs in Mississauga.
Haridwar ❤CALL Girls 🔝 89011★83002 🔝 ❤ℂall Girls IN Haridwar ESCORT SERVICE❤

Haridwar ❤CALL Girls 🔝 89011★83002 🔝 ❤ℂall Girls IN Haridwar ESCORT SERVICE❤
Artificial Intelligence to Optimize Cardiovascular Therapy

Presentation at the annual convention of the Philippine Heart Association, 31 May 2024. EDSA Shangrila Hotel, Manila.
ABDOMINAL COMPARTMENT SYSNDROME

CARE OF CLIENTS WITH LIFE-THREATENING CONDITIONS, ACUTELY ILL/ MULTI-ORGAN PROBLEMS, HIGH ACUITY AND EMERGENCY SITUATION
Medical Technology Tackles New Health Care Demand - Research Report - March 2...

M Capital Group (“MCG”) predicts that with, against, despite, and even without the global pandemic, the medical technology (MedTech) industry shows signs of continuous healthy growth, driven by smaller, faster, and cheaper devices, growing demand for home-based applications, technological innovation, strategic acquisitions, investments, and SPAC listings. MCG predicts that this should reflects itself in annual growth of over 6%, well beyond 2028.
According to Chris Mouchabhani, Managing Partner at M Capital Group, “Despite all economic scenarios that one may consider, beyond overall economic shocks, medical technology should remain one of the most promising and robust sectors over the short to medium term and well beyond 2028.”
There is a movement towards home-based care for the elderly, next generation scanning and MRI devices, wearable technology, artificial intelligence incorporation, and online connectivity. Experts also see a focus on predictive, preventive, personalized, participatory, and precision medicine, with rising levels of integration of home care and technological innovation.
The average cost of treatment has been rising across the board, creating additional financial burdens to governments, healthcare providers and insurance companies. According to MCG, cost-per-inpatient-stay in the United States alone rose on average annually by over 13% between 2014 to 2021, leading MedTech to focus research efforts on optimized medical equipment at lower price points, whilst emphasizing portability and ease of use. Namely, 46% of the 1,008 medical technology companies in the 2021 MedTech Innovator (“MTI”) database are focusing on prevention, wellness, detection, or diagnosis, signaling a clear push for preventive care to also tackle costs.
In addition, there has also been a lasting impact on consumer and medical demand for home care, supported by the pandemic. Lockdowns, closure of care facilities, and healthcare systems subjected to capacity pressure, accelerated demand away from traditional inpatient care. Now, outpatient care solutions are driving industry production, with nearly 70% of recent diagnostics start-up companies producing products in areas such as ambulatory clinics, at-home care, and self-administered diagnostics.
GURGAON Call Girls ❤8901183002❤ #ℂALL# #gIRLS# In GURGAON ₹,2500 Cash Payment...

GURGAON Call Girls ❤8901183002❤ #ℂALL# #gIRLS# In GURGAON ₹,2500 Cash Payment Free Home Delivery (DELHI) ESCORT
Roti bank chennai PPT [Autosaved].pptx1![Roti bank chennai PPT [Autosaved].pptx1](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![Roti bank chennai PPT [Autosaved].pptx1](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
One of the most developed cities of India, the city of Chennai is the capital of Tamilnadu and many people from different parts of India come here to earn their bread and butter. Being a metropolitan, the city is filled with towering building and beaches but the sad part as with almost every Indian city
Recently uploaded (20)
VERIFICATION AND VALIDATION TOOLKIT Determining Performance Characteristics o...

VERIFICATION AND VALIDATION TOOLKIT Determining Performance Characteristics o...
the IUA Administrative Board and General Assembly meeting

the IUA Administrative Board and General Assembly meeting
India Clinical Trials Market: Industry Size and Growth Trends [2030] Analyzed...![India Clinical Trials Market: Industry Size and Growth Trends [2030] Analyzed...](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
![India Clinical Trials Market: Industry Size and Growth Trends [2030] Analyzed...](data:image/gif;base64,R0lGODlhAQABAIAAAAAAAP///yH5BAEAAAAALAAAAAABAAEAAAIBRAA7)
India Clinical Trials Market: Industry Size and Growth Trends [2030] Analyzed...
CHAPTER 1 SEMESTER V - ROLE OF PEADIATRIC NURSE.pdf

CHAPTER 1 SEMESTER V - ROLE OF PEADIATRIC NURSE.pdf
VVIP Dehradun Girls 9719300533 Heat-bake { Dehradun } Genteel ℂall Serviℂe By...

VVIP Dehradun Girls 9719300533 Heat-bake { Dehradun } Genteel ℂall Serviℂe By...
Navigating Women's Health: Understanding Prenatal Care and Beyond

Navigating Women's Health: Understanding Prenatal Care and Beyond
Haridwar ❤CALL Girls 🔝 89011★83002 🔝 ❤ℂall Girls IN Haridwar ESCORT SERVICE❤

Haridwar ❤CALL Girls 🔝 89011★83002 🔝 ❤ℂall Girls IN Haridwar ESCORT SERVICE❤
Artificial Intelligence to Optimize Cardiovascular Therapy

Artificial Intelligence to Optimize Cardiovascular Therapy
Medical Technology Tackles New Health Care Demand - Research Report - March 2...

Medical Technology Tackles New Health Care Demand - Research Report - March 2...
GURGAON Call Girls ❤8901183002❤ #ℂALL# #gIRLS# In GURGAON ₹,2500 Cash Payment...

GURGAON Call Girls ❤8901183002❤ #ℂALL# #gIRLS# In GURGAON ₹,2500 Cash Payment...
Surgery-Mini-OSCE-All-Past-Years-Questions-Modified.

Surgery-Mini-OSCE-All-Past-Years-Questions-Modified.
Europe IVD medical registration and approval chart - EMERGO
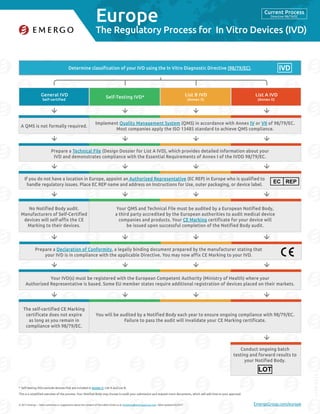
- 1. Europe © 2017 Emergo – Have comments or suggestions about the content of this table? Email us at marketing@emergogroup.com. Table updated 02/2017. EmergoGroup.com/europe Determine classification of your IVD using the In Vitro Diagnostic Directive (98/79/EC). General IVD Self-certified Self-Testing IVD* List B IVD (Annex II) List A IVD (Annex II) 5175-0217 The Regulatory Process for In Vitro Devices (IVD) Self-testing IVDs exclude devices that are included in Annex II, List A and List B. This is a simplified overview of the process. Your Notified Body may choose to audit your submission and request more documents, which will add time to your approval. Implement Quality Management System (QMS) in accordance with Annex IV or VII of 98/79/EC. Most companies apply the ISO 13485 standard to achieve QMS compliance. A QMS is not formally required. Your QMS and Technical File must be audited by a European Notified Body, a third party accredited by the European authorities to audit medical device companies and products. Your CE Marking certificate for your device will be issued upon successful completion of the Notified Body audit. No Notified Body audit. Manufacturers of Self-Certified devices will self-affix the CE Marking to their devices. You will be audited by a Notified Body each year to ensure ongoing compliance with 98/79/EC. Failure to pass the audit will invalidate your CE Marking certificate. The self-certified CE Marking certificate does not expire as long as you remain in compliance with 98/79/EC. Prepare a Technical File (Design Dossier for List A IVD), which provides detailed information about your IVD and demonstrates compliance with the Essential Requirements of Annex I of the IVDD 98/79/EC. Your IVD(s) must be registered with the European Competent Authority (Ministry of Health) where your Authorized Representative is based. Some EU member states require additional registration of devices placed on their markets. If you do not have a location in Europe, appoint an Authorized Representative (EC REP) in Europe who is qualified to handle regulatory issues. Place EC REP name and address on Instructions for Use, outer packaging, or device label. EC REP Prepare a Declaration of Conformity, a legally binding document prepared by the manufacturer stating that your IVD is in compliance with the applicable Directive. You may now affix CE Marking to your IVD. * IVD Conduct ongoing batch testing and forward results to your Notified Body. LOT Current Process Directive 98/79/EC
- 2. Europe Notes 1. The time frames shown above are typical for the majority of IVD submissions but assume that your device does not contain animal tissue, medicinal substances or employ entirely novel technology. Your length of approval will depend on the quality and completeness of your technical documentation and how much time you take to address additional information requests from authorities after submission. YOUR SUBMISSION(S) MAY TAKE MORE TIME THAN WHAT IS SHOWN ABOVE. 2. Registrations remain valid for the time specified as long as you do not make changes to your IVD, intended use or indications for use. 3. We recommend starting the re-registration process no later than the time period specified above. However, please consult with your distributor or regulatory expert well before this suggested time to avoid any lapse in your registration. 4. Our rating of the complexity of the registration process is based on our experience and the opinion of nearly 1,000 QA/RA professionals worldwide who were asked to rate the difficulty of registering a device in each country. The European CE Marking process is considered the mid-point to which all other markets are compared. 5. Prices in US Dollars for a single IVD device. 1 = Less than $5,000; 2 = $10,000 - $15,000; 3 = $15,000 - $30,000; 4 = $30,000 - $50,000; 5 = $50,000 or more. Estimated cost includes registration application fees, in-country representation, Notified Body audit fees, submission preparation consulting and translation of documents, if required. Costs assume you already have approval for your device in the United States, Canada, Australia, or Japan. Costs do NOT include product testing, clinical trials or ISO 13485 implementation, if applicable. These costs are generally spread across many markets and thus not specifically attributed to a European CE submission. EmergoGroup.com/europe 5175-0217 © 2017 Emergo – Have comments or suggestions about the content of this table? Email us at marketing@emergogroup.com. Table updated 02/2017. Time, Cost, and Complexity of IVD Registration Self-testing IVDs exclude devices that are included in Annex II, List A and List B. This is a simplified overview of the process. Your Notified Body may choose to audit your submission and request more documents, which will add time to your approval. * Device classification in Europe General IVD Self-certified Self-Testing IVD* List B IVD (Annex II) List A IVD (Annex II) How long you should expect to wait after submission until approval is granted.1 Not applicable 3-5 months 3-5 months 9-12 months Validity period for CE Marking certificate.2 Not applicable 3 years 3 years 3 years Registration renewal should be started this far in advance.3 Not applicable 2 months 2 months 2 months Complexity of the registration process for this classification.4 Overall cost of gaining regulatory approval.5 Simple Complex Simple Complex Simple Complex Simple Complex Low High Low High Low High Low High 1 1 2 3 3 4 44 Current Process Directive 98/79/EC
