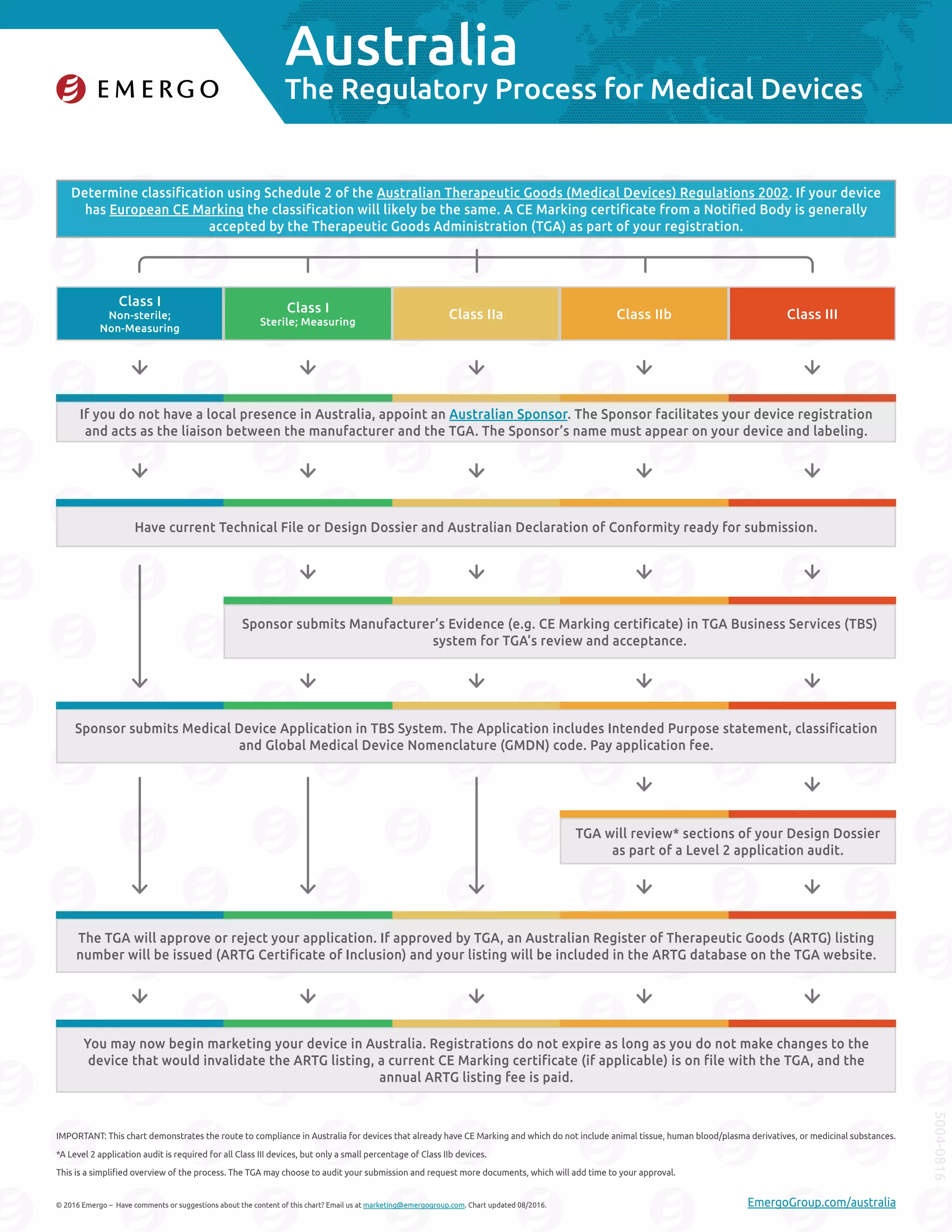
1. To register a medical device in Australia, manufacturers must first determine the device's classification using Australia's regulations and submit an application through an Australian sponsor.
2. The sponsor will submit the application along with supporting documents to the Therapeutic Goods Administration (TGA) for review and approval.
3. If approved, the TGA will provide an Australian Register of Therapeutic Goods (ARTG) listing number allowing marketing in Australia. Listings do not expire as long as annual fees are paid and the device is not changed.