South Korea medical device approval chart - Emergo
•
2 likes•4,968 views
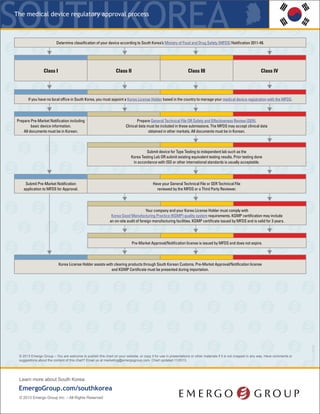
The regulatory process for medical devices in South Korea requires manufacturers to determine the classification of their device and submit the appropriate registration application and supporting documentation to the Ministry of Food and Drug Safety (MFDS). For new devices without a predicate, manufacturers must submit clinical data as part of a Clinical Data Review. Class II devices considered "Special Equivalent" have an expediated review process without a Technical File requirement. Prior international testing may satisfy some requirements but additional Korea-specific testing is often necessary. The MFDS may request additional information or audit submissions, extending approval timelines.
Report
Share
Report
Share
Download to read offline
Recommended
Brazil medical device registration and approval chart - EMERGO

Brazil has a classification system for medical devices with four classes - I, II, III, and IV. Class I and II devices require a simplified registration called a Cadastro, while Class III and IV devices require a more rigorous registration called a Registro. To register a device, a company must appoint a Brazil Registration Holder to submit the application to ANVISA and obtain necessary approvals. Certain devices may also require certification from inspection agency INMETRO. The registration process can take 1-3 months for Classes I and II or 8-15 months for Classes III and IV, though Class III and IV can take over 4 years if additional inspections are required.
Europe CE Marking for medical devices under new MDR

Starting in early 2020, medical devices seeking CE Marking certification in Europe must comply with the new Medical Device Regulation (MDR). This will require appointing personnel responsible for regulatory compliance, classifying the device, appointing an Authorized Representative located in the EU, including labeling with this information, and obtaining a Single Registration Number. Manufacturers must also prepare technical documentation, implement a quality management system, and undergo annual audits by a Notified Body to maintain certification.
Medical device approval chart for Russia - EMERGO

The regulatory process for medical devices in Russia involves classifying the device, appointing an authorized representative, submitting a registration dossier including technical documentation and clinical data to Roszdravnadzor (RZN), addressing any requests for additional information, and ultimately receiving a registration certificate to market the device in Russia. The process can take 6-12 months depending on the device class.
Canada medical device approval chart - EMERGO

1. To market medical devices in Canada, manufacturers must classify their device according to regulations, implement an ISO 13485 quality management system with additional Canadian requirements, and obtain ISO 13485 certification from an accredited registrar.
2. Manufacturers then prepare license applications, supporting documents, and pay fees, with process times ranging from 1-8 months depending on device class.
3. Licenses must be renewed annually by meeting regulatory requirements and paying fees to avoid revocation.
Taiwan medical device registration and approval chart - EMERGO

Taiwan regulates medical devices through the Pharmaceutical Affairs Act and Regulations for Governing the Management of Medical Devices. The process involves classifying the device, appointing a Taiwan agent, preparing quality system documentation for submission, and obtaining approval. Device classification and complexity of approval requirements vary, with Class I generally having the simplest process taking 1-2 months, Class II taking 10-12 months, and Class III taking 10-12 months and requiring a committee review.
India medical device regulatory process

The regulatory framework for medical devices in India is based on drug regulations under the Drugs and Cosmetics Act of 1940 and Drugs and Cosmetics Rules of 1945. The Drug Controller General of India (DCGI) within the Central Drugs Standard Control Organization (CDSCO) regulates medical devices and IVDs. Currently only a limited number of medical device and IVD products require registration in India, including ablation devices, dental implants, and hernia mesh. The registration process for notified devices can take 9-12 months and involves appointing an authorized agent, compiling an application, and obtaining CDSCO approval.
Japan medical device approval chart - Emergo 

The regulatory process for medical devices in Japan involves several key steps:
1) Classifying the device and appointing a Marketing Authorization Holder who will manage the registration process.
2) Implementing a Quality Management System that complies with relevant regulations.
3) Submitting an application to either the Pharmaceuticals and Medical Devices Agency or a Registered Certification Body, depending on the device class.
4) Undergoing an audit and receiving the appropriate certificate before marketing the device in Japan. Device registrations do not expire but must be renewed periodically.
China medical device approval chart - EMERGO

1. The document outlines the regulatory process for medical devices in China, including classification of devices and the approval process for Class I, II, and III devices.
2. Class I devices have the simplest approval process, requiring only an administrative review with no submission fees. Class II and III devices require more extensive technical documentation, testing in China, and clinical evaluations.
3. The approval process can take 12-22 months for Class II and III devices and involves appointing a Chinese agent, submitting documentation and application materials in Chinese, testing, and a review by the China Food and Drug Administration before a registration certificate is issued.
Recommended
Brazil medical device registration and approval chart - EMERGO

Brazil has a classification system for medical devices with four classes - I, II, III, and IV. Class I and II devices require a simplified registration called a Cadastro, while Class III and IV devices require a more rigorous registration called a Registro. To register a device, a company must appoint a Brazil Registration Holder to submit the application to ANVISA and obtain necessary approvals. Certain devices may also require certification from inspection agency INMETRO. The registration process can take 1-3 months for Classes I and II or 8-15 months for Classes III and IV, though Class III and IV can take over 4 years if additional inspections are required.
Europe CE Marking for medical devices under new MDR

Starting in early 2020, medical devices seeking CE Marking certification in Europe must comply with the new Medical Device Regulation (MDR). This will require appointing personnel responsible for regulatory compliance, classifying the device, appointing an Authorized Representative located in the EU, including labeling with this information, and obtaining a Single Registration Number. Manufacturers must also prepare technical documentation, implement a quality management system, and undergo annual audits by a Notified Body to maintain certification.
Medical device approval chart for Russia - EMERGO

The regulatory process for medical devices in Russia involves classifying the device, appointing an authorized representative, submitting a registration dossier including technical documentation and clinical data to Roszdravnadzor (RZN), addressing any requests for additional information, and ultimately receiving a registration certificate to market the device in Russia. The process can take 6-12 months depending on the device class.
Canada medical device approval chart - EMERGO

1. To market medical devices in Canada, manufacturers must classify their device according to regulations, implement an ISO 13485 quality management system with additional Canadian requirements, and obtain ISO 13485 certification from an accredited registrar.
2. Manufacturers then prepare license applications, supporting documents, and pay fees, with process times ranging from 1-8 months depending on device class.
3. Licenses must be renewed annually by meeting regulatory requirements and paying fees to avoid revocation.
Taiwan medical device registration and approval chart - EMERGO

Taiwan regulates medical devices through the Pharmaceutical Affairs Act and Regulations for Governing the Management of Medical Devices. The process involves classifying the device, appointing a Taiwan agent, preparing quality system documentation for submission, and obtaining approval. Device classification and complexity of approval requirements vary, with Class I generally having the simplest process taking 1-2 months, Class II taking 10-12 months, and Class III taking 10-12 months and requiring a committee review.
India medical device regulatory process

The regulatory framework for medical devices in India is based on drug regulations under the Drugs and Cosmetics Act of 1940 and Drugs and Cosmetics Rules of 1945. The Drug Controller General of India (DCGI) within the Central Drugs Standard Control Organization (CDSCO) regulates medical devices and IVDs. Currently only a limited number of medical device and IVD products require registration in India, including ablation devices, dental implants, and hernia mesh. The registration process for notified devices can take 9-12 months and involves appointing an authorized agent, compiling an application, and obtaining CDSCO approval.
Japan medical device approval chart - Emergo 

The regulatory process for medical devices in Japan involves several key steps:
1) Classifying the device and appointing a Marketing Authorization Holder who will manage the registration process.
2) Implementing a Quality Management System that complies with relevant regulations.
3) Submitting an application to either the Pharmaceuticals and Medical Devices Agency or a Registered Certification Body, depending on the device class.
4) Undergoing an audit and receiving the appropriate certificate before marketing the device in Japan. Device registrations do not expire but must be renewed periodically.
China medical device approval chart - EMERGO

1. The document outlines the regulatory process for medical devices in China, including classification of devices and the approval process for Class I, II, and III devices.
2. Class I devices have the simplest approval process, requiring only an administrative review with no submission fees. Class II and III devices require more extensive technical documentation, testing in China, and clinical evaluations.
3. The approval process can take 12-22 months for Class II and III devices and involves appointing a Chinese agent, submitting documentation and application materials in Chinese, testing, and a review by the China Food and Drug Administration before a registration certificate is issued.
Australia medical device registration and approval process - EMERGO

1. To register a medical device in Australia, manufacturers must first determine the device's classification using Australia's regulations and submit an application through an Australian sponsor.
2. The sponsor will submit the application along with supporting documents to the Therapeutic Goods Administration (TGA) for review and approval.
3. If approved, the TGA will provide an Australian Register of Therapeutic Goods (ARTG) listing number allowing marketing in Australia. Listings do not expire as long as annual fees are paid and the device is not changed.
US FDA medical device approval chart - Emergo 

Simple one page chart shows the US FDA medical device approval process for Class 1, 2 and 3 devices. Very easy to understand.
Saudi arabia medical device regulatory process

To market medical devices in Saudi Arabia, devices must receive marketing authorization from the Saudi Food and Drug Authority (SFDA). The SFDA application is reviewed for completeness then sent to a third party for technical review, which can involve multiple rounds of questions. Once approved, the SFDA issues a certificate allowing marketing in Saudi Arabia. Appointing an authorized local representative is also required to manage the registration process. Overall, registration times range from 1-6 months but costs and complexity vary depending on the device's risk classification.
Medical device approval chart for Mexico - Emergo 

In Mexico, medical devices and in-vitro diagnostic devices are regulated by COFEPRIS, a division of the Mexican Ministry of Health. The regulatory process involves classifying the device, appointing a Mexico Registration Holder representative, preparing a registration dossier in Spanish including documentation of quality management and technical specifications, and submitting the application to COFEPRIS for review. Approval time ranges from 1-10 months depending on the device class and review process. Registrations are valid for 5 years and must be renewed in advance of the expiration date.
Colombia medical device approval chart - Emergo 

The Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA) governs medical devices in Colombia. To register a device, a manufacturer must determine its classification, provide documentation such as quality and safety certificates, and submit an application through an Importer or Legal Representative. Approval times range from automatic for Class I and IIa devices to 4-6 months for Class IIb and III. Once approved, registrations are valid for 10 years.
Europe IVD medical registration and approval chart - EMERGO

The document summarizes the regulatory process for in vitro diagnostic devices (IVDs) in Europe under the In Vitro Diagnostic Directive (98/79/EC). It outlines the classification of IVDs, requirements for quality management systems and technical files, roles of notified bodies and authorized representatives, and timelines and costs associated with the approval process depending on the IVD classification. The process can take from 3-5 months for self-certified IVDs to 9-12 months for list A IVDs and involves implementing quality systems, obtaining notified body audits, and registering with authorities.
Japan PMDA Medical Device Regulatory Approval Process

Watch the recorded webinar: https://www.emergogroup.com/resources/video-webinar-japan-registration-process
Japan's medical device market is one of the most robust markets in Asia, but its regulatory system can be confusing. Before deciding to sell your device in the Japanese market, it is important to understand how the regulations apply to your device, which steps to take, and what resources are required to complete the process. In this presentation, Ann Marie Boullie, Vice President of Business Development for EMERGO, will discuss some of the most complex aspects of the Japanese registration process, including:
JMDN codes: device classification and predicates
Clinical data requirements and PMDA pre-submission meetings
Registration routes (Todokede, Ninsho, Shonin)
QMS (Ordinance 169) requirements
Role of the Marketing Authorization Holder (MAH)
Plus more ...
Japan Medical Device Regulatory Approval Process

Before deciding to sell your device in the Japanese market, it is important to understand how the regulations apply to your device, which steps to take, and what resources are required to complete the process. In this presentation, Ann Marie Boullie, Vice President of Business Development for EMERGO, outlines some of the most complex aspects of the Japanese registration process, including:
JMDN codes: device classification and predicates
Clinical data requirements and PMDA pre-submission meetings
Registration routes (Todokede, Ninsho, Shonin)
QMS (Ordinance 169) requirements
Role of the Marketing Authorization Holder (MAH)
China: Medical Device Regulations

This whitepaper provides an overview of Chinese Medical Device Regulations. This includes an overview of the Chinese medical device market, medical device regulatory authorities, medical device registration procedure and medical device classification. It also provides information on regulations regarding product standard, type testing, and clinical trials. This paper is meant for anyone within the regulatory affairs industry who is looking to learn more about medical device regulations and product registration in China.
For more information, contact us for a free 15 minute consultation at http://www.pacificbridgemedical.com/contact-us/.
Prior Approval Supplements (PAS)

This presentation explains in brief the process, types, requirements, and conditions where PAS is needed to submit by drug manufacturers.
Regulatory approval process for invitro diagnostics in us

This document summarizes the regulatory approval process for in vitro diagnostics (IVDs) in the United States. IVDs are classified into Class I, II, or III based on risk, with Class III devices requiring premarket approval. The main regulatory pathways for approval are 510(k) premarket notification for demonstrating substantial equivalence to a predicate device or the premarket approval (PMA) process for novel high-risk devices. Clinical Laboratory Improvement Amendments also provide quality standards for lab testing. The document reviews the classification system and options for 510(k), PMA, de novo, and lab developed tests.
The EU’s Medical Device Regulation

The EU’s medical device regulation
Medical device manufacturers seeking market access
to the European Union (EU) will soon face major changes
in the EU’s decades-old regulatory framework. The EU’s
Medical Device Regulation (MDR) was officially published
on 5 May 2017 and came into force on 25 May 2017.
The MDR will replace the EU’s current Medical Device
Directive (93/42/EEC) and the EU’s Directive on active
implantable medical devices (90/385/EEC).
Canadaapprovalprocess final13june2012-130116090730-phpapp01

The document discusses the medical device registration process in Canada. It outlines the key steps, which include determining the device classification, obtaining necessary licenses for manufacturers and distributors, implementing quality systems according to ISO 13485, undergoing regulatory audits, and preparing required documentation for premarket review. The process varies depending on the risk class of the device and whether the applicant is a manufacturer, distributor or importer.
Regulation of medical device in japan

Medical Devices registration in Japan , quality system requirements and evaluation and investigation of medical devices in regulated countries ASEAN, China JAPAN and WHO regulations. quality and ethical considerations regulatory and documentation requirements for marketing medical devices and IVDs in regulated countries.
The regulation of medical devices in Australia

The regulation of medical devices in Australia involves classifying devices based on their intended use and risk level. Higher risk devices undergo more rigorous assessment procedures to ensure they meet essential safety and performance principles before being approved for market. Ongoing monitoring is also conducted after devices enter the market to protect public health. The TGA regulates medical devices to confirm they are suitable for their intended purpose and that their benefits outweigh any risks when used correctly.
Presentation: Software as a Medical Device: Regulatory insights and Q & A

The document provides an overview of the Therapeutic Goods Administration (TGA) in Australia, which regulates medical devices and software. It discusses:
- The TGA evaluates medical devices before and after market to ensure safety, quality and performance.
- Medical devices are classified based on risk from Class I to III, with Class III requiring the most oversight and pre-market evaluation.
- All medical devices must comply with the Essential Principles which address design, safety and intended use. Higher risk devices require more regulatory procedures.
- Software can be regulated as a medical device (Software as a Medical Device or SaMD) if it meets the definition and new rules are being proposed for SaMD in Australia
Regulatory requirements for CE CERTIFICATION of Medical Devices in European U...

The EU introduced the CE marking scheme to make trade easier and cheaper between EU countries. It means that a manufacturer claims that their product conforms to the minimum legal requirements for health and safety as laid down in EU directives. In addition, it may be considered a benefit that by implementing the requirements, the product will be safer for the user and this may also reduce damage and liability claims. Additional benefits may include your product being made safer for end-users. The EU introduced the CE marking scheme to make trade easier and cheaper between EU countries. It means that a manufacturer claims that their product conforms to the minimum legal requirements for health and safety as laid down in EU directives. If you manufacture or import a product which falls within the scope of one or more of the New Approach Directives and wish to place your product on the market in any of the member’s states of the European Economic Area (EEA), then you must apply CE marking to your product against the essential requirements of all these applicable directives.
Key Words: European Union, CE marking, New Approach Directives, EEA, Regulatory requirements.
Devices Sponsor Information Day: 3A - Medical Devices - Manufacturer's eviden...

Presentations by TGA and Industry (combined) to help sponsors and manufacturers better understand the regulation of medical devices and in-vitro diagnostic medical devices
6 medical device registration in singapore

The document discusses medical device registration requirements in Singapore. The Health Sciences Authority (HSA) regulates medical devices and requires all devices to be registered prior to placement on the Singapore market, except for some low-risk Class A devices. Medical device dealers must be registered with the Accounting and Corporate Regulatory Authority (ACRA). Devices are classified from Class A to D based on risk, with higher classes facing more stringent registration routes including full evaluation. Registration application and evaluation fees vary based on device class and route, and processing times range from 30 days for some Class A devices to over 300 days for certain Class D devices.
validation and verification of medical device.pptx

M.Pharm Regulatory Affairs
2nd Sem
Subject: Regulatory Aspects of Medical devices.
Topic: Validation and Verification of Medical devices.
Pakistan Medical Device regulatory process

Medical devices in Pakistan are regulated by the Medical Device & Medicated Cosmetics Division and must be classified according to the Medical Device Rules of 2017. The regulatory process involves appointing a registered importer, determining the classification, and having an authorized representative submit an application dossier and fees to the Medical Devices Board for approval. Approval times range from less than two months for Class A local manufacturer devices to 8-10 months for Class D imported devices not from reference countries.
RDSO Guidelines for new Vendors- Rajni Ranjan

This document provides guidelines for vendor development and approval for small track machines and tools by the Research Designs and Standards Organisation (RDSO) of the Government of India Ministry of Railways.
It defines key terms, outlines the policy and process for vendor registration and approval, including application assessment, technical capability assessment, prototype testing, and approval procedures. It also covers charges for various activities, validity of approvals, and requirements for new items or extensions. The Standing Committee on Small Track Machines examines cases and makes recommendations to the Railway Board for final approval.
More Related Content
What's hot
Australia medical device registration and approval process - EMERGO

1. To register a medical device in Australia, manufacturers must first determine the device's classification using Australia's regulations and submit an application through an Australian sponsor.
2. The sponsor will submit the application along with supporting documents to the Therapeutic Goods Administration (TGA) for review and approval.
3. If approved, the TGA will provide an Australian Register of Therapeutic Goods (ARTG) listing number allowing marketing in Australia. Listings do not expire as long as annual fees are paid and the device is not changed.
US FDA medical device approval chart - Emergo 

Simple one page chart shows the US FDA medical device approval process for Class 1, 2 and 3 devices. Very easy to understand.
Saudi arabia medical device regulatory process

To market medical devices in Saudi Arabia, devices must receive marketing authorization from the Saudi Food and Drug Authority (SFDA). The SFDA application is reviewed for completeness then sent to a third party for technical review, which can involve multiple rounds of questions. Once approved, the SFDA issues a certificate allowing marketing in Saudi Arabia. Appointing an authorized local representative is also required to manage the registration process. Overall, registration times range from 1-6 months but costs and complexity vary depending on the device's risk classification.
Medical device approval chart for Mexico - Emergo 

In Mexico, medical devices and in-vitro diagnostic devices are regulated by COFEPRIS, a division of the Mexican Ministry of Health. The regulatory process involves classifying the device, appointing a Mexico Registration Holder representative, preparing a registration dossier in Spanish including documentation of quality management and technical specifications, and submitting the application to COFEPRIS for review. Approval time ranges from 1-10 months depending on the device class and review process. Registrations are valid for 5 years and must be renewed in advance of the expiration date.
Colombia medical device approval chart - Emergo 

The Instituto Nacional de Vigilancia de Medicamentos y Alimentos (INVIMA) governs medical devices in Colombia. To register a device, a manufacturer must determine its classification, provide documentation such as quality and safety certificates, and submit an application through an Importer or Legal Representative. Approval times range from automatic for Class I and IIa devices to 4-6 months for Class IIb and III. Once approved, registrations are valid for 10 years.
Europe IVD medical registration and approval chart - EMERGO

The document summarizes the regulatory process for in vitro diagnostic devices (IVDs) in Europe under the In Vitro Diagnostic Directive (98/79/EC). It outlines the classification of IVDs, requirements for quality management systems and technical files, roles of notified bodies and authorized representatives, and timelines and costs associated with the approval process depending on the IVD classification. The process can take from 3-5 months for self-certified IVDs to 9-12 months for list A IVDs and involves implementing quality systems, obtaining notified body audits, and registering with authorities.
Japan PMDA Medical Device Regulatory Approval Process

Watch the recorded webinar: https://www.emergogroup.com/resources/video-webinar-japan-registration-process
Japan's medical device market is one of the most robust markets in Asia, but its regulatory system can be confusing. Before deciding to sell your device in the Japanese market, it is important to understand how the regulations apply to your device, which steps to take, and what resources are required to complete the process. In this presentation, Ann Marie Boullie, Vice President of Business Development for EMERGO, will discuss some of the most complex aspects of the Japanese registration process, including:
JMDN codes: device classification and predicates
Clinical data requirements and PMDA pre-submission meetings
Registration routes (Todokede, Ninsho, Shonin)
QMS (Ordinance 169) requirements
Role of the Marketing Authorization Holder (MAH)
Plus more ...
Japan Medical Device Regulatory Approval Process

Before deciding to sell your device in the Japanese market, it is important to understand how the regulations apply to your device, which steps to take, and what resources are required to complete the process. In this presentation, Ann Marie Boullie, Vice President of Business Development for EMERGO, outlines some of the most complex aspects of the Japanese registration process, including:
JMDN codes: device classification and predicates
Clinical data requirements and PMDA pre-submission meetings
Registration routes (Todokede, Ninsho, Shonin)
QMS (Ordinance 169) requirements
Role of the Marketing Authorization Holder (MAH)
China: Medical Device Regulations

This whitepaper provides an overview of Chinese Medical Device Regulations. This includes an overview of the Chinese medical device market, medical device regulatory authorities, medical device registration procedure and medical device classification. It also provides information on regulations regarding product standard, type testing, and clinical trials. This paper is meant for anyone within the regulatory affairs industry who is looking to learn more about medical device regulations and product registration in China.
For more information, contact us for a free 15 minute consultation at http://www.pacificbridgemedical.com/contact-us/.
Prior Approval Supplements (PAS)

This presentation explains in brief the process, types, requirements, and conditions where PAS is needed to submit by drug manufacturers.
Regulatory approval process for invitro diagnostics in us

This document summarizes the regulatory approval process for in vitro diagnostics (IVDs) in the United States. IVDs are classified into Class I, II, or III based on risk, with Class III devices requiring premarket approval. The main regulatory pathways for approval are 510(k) premarket notification for demonstrating substantial equivalence to a predicate device or the premarket approval (PMA) process for novel high-risk devices. Clinical Laboratory Improvement Amendments also provide quality standards for lab testing. The document reviews the classification system and options for 510(k), PMA, de novo, and lab developed tests.
The EU’s Medical Device Regulation

The EU’s medical device regulation
Medical device manufacturers seeking market access
to the European Union (EU) will soon face major changes
in the EU’s decades-old regulatory framework. The EU’s
Medical Device Regulation (MDR) was officially published
on 5 May 2017 and came into force on 25 May 2017.
The MDR will replace the EU’s current Medical Device
Directive (93/42/EEC) and the EU’s Directive on active
implantable medical devices (90/385/EEC).
Canadaapprovalprocess final13june2012-130116090730-phpapp01

The document discusses the medical device registration process in Canada. It outlines the key steps, which include determining the device classification, obtaining necessary licenses for manufacturers and distributors, implementing quality systems according to ISO 13485, undergoing regulatory audits, and preparing required documentation for premarket review. The process varies depending on the risk class of the device and whether the applicant is a manufacturer, distributor or importer.
Regulation of medical device in japan

Medical Devices registration in Japan , quality system requirements and evaluation and investigation of medical devices in regulated countries ASEAN, China JAPAN and WHO regulations. quality and ethical considerations regulatory and documentation requirements for marketing medical devices and IVDs in regulated countries.
The regulation of medical devices in Australia

The regulation of medical devices in Australia involves classifying devices based on their intended use and risk level. Higher risk devices undergo more rigorous assessment procedures to ensure they meet essential safety and performance principles before being approved for market. Ongoing monitoring is also conducted after devices enter the market to protect public health. The TGA regulates medical devices to confirm they are suitable for their intended purpose and that their benefits outweigh any risks when used correctly.
Presentation: Software as a Medical Device: Regulatory insights and Q & A

The document provides an overview of the Therapeutic Goods Administration (TGA) in Australia, which regulates medical devices and software. It discusses:
- The TGA evaluates medical devices before and after market to ensure safety, quality and performance.
- Medical devices are classified based on risk from Class I to III, with Class III requiring the most oversight and pre-market evaluation.
- All medical devices must comply with the Essential Principles which address design, safety and intended use. Higher risk devices require more regulatory procedures.
- Software can be regulated as a medical device (Software as a Medical Device or SaMD) if it meets the definition and new rules are being proposed for SaMD in Australia
Regulatory requirements for CE CERTIFICATION of Medical Devices in European U...

The EU introduced the CE marking scheme to make trade easier and cheaper between EU countries. It means that a manufacturer claims that their product conforms to the minimum legal requirements for health and safety as laid down in EU directives. In addition, it may be considered a benefit that by implementing the requirements, the product will be safer for the user and this may also reduce damage and liability claims. Additional benefits may include your product being made safer for end-users. The EU introduced the CE marking scheme to make trade easier and cheaper between EU countries. It means that a manufacturer claims that their product conforms to the minimum legal requirements for health and safety as laid down in EU directives. If you manufacture or import a product which falls within the scope of one or more of the New Approach Directives and wish to place your product on the market in any of the member’s states of the European Economic Area (EEA), then you must apply CE marking to your product against the essential requirements of all these applicable directives.
Key Words: European Union, CE marking, New Approach Directives, EEA, Regulatory requirements.
Devices Sponsor Information Day: 3A - Medical Devices - Manufacturer's eviden...

Presentations by TGA and Industry (combined) to help sponsors and manufacturers better understand the regulation of medical devices and in-vitro diagnostic medical devices
6 medical device registration in singapore

The document discusses medical device registration requirements in Singapore. The Health Sciences Authority (HSA) regulates medical devices and requires all devices to be registered prior to placement on the Singapore market, except for some low-risk Class A devices. Medical device dealers must be registered with the Accounting and Corporate Regulatory Authority (ACRA). Devices are classified from Class A to D based on risk, with higher classes facing more stringent registration routes including full evaluation. Registration application and evaluation fees vary based on device class and route, and processing times range from 30 days for some Class A devices to over 300 days for certain Class D devices.
validation and verification of medical device.pptx

M.Pharm Regulatory Affairs
2nd Sem
Subject: Regulatory Aspects of Medical devices.
Topic: Validation and Verification of Medical devices.
What's hot (20)
Australia medical device registration and approval process - EMERGO

Australia medical device registration and approval process - EMERGO
Europe IVD medical registration and approval chart - EMERGO

Europe IVD medical registration and approval chart - EMERGO
Japan PMDA Medical Device Regulatory Approval Process

Japan PMDA Medical Device Regulatory Approval Process
Regulatory approval process for invitro diagnostics in us

Regulatory approval process for invitro diagnostics in us
Canadaapprovalprocess final13june2012-130116090730-phpapp01

Canadaapprovalprocess final13june2012-130116090730-phpapp01
Presentation: Software as a Medical Device: Regulatory insights and Q & A

Presentation: Software as a Medical Device: Regulatory insights and Q & A
Regulatory requirements for CE CERTIFICATION of Medical Devices in European U...

Regulatory requirements for CE CERTIFICATION of Medical Devices in European U...
Devices Sponsor Information Day: 3A - Medical Devices - Manufacturer's eviden...

Devices Sponsor Information Day: 3A - Medical Devices - Manufacturer's eviden...
validation and verification of medical device.pptx

validation and verification of medical device.pptx
Similar to South Korea medical device approval chart - Emergo
Pakistan Medical Device regulatory process

Medical devices in Pakistan are regulated by the Medical Device & Medicated Cosmetics Division and must be classified according to the Medical Device Rules of 2017. The regulatory process involves appointing a registered importer, determining the classification, and having an authorized representative submit an application dossier and fees to the Medical Devices Board for approval. Approval times range from less than two months for Class A local manufacturer devices to 8-10 months for Class D imported devices not from reference countries.
RDSO Guidelines for new Vendors- Rajni Ranjan

This document provides guidelines for vendor development and approval for small track machines and tools by the Research Designs and Standards Organisation (RDSO) of the Government of India Ministry of Railways.
It defines key terms, outlines the policy and process for vendor registration and approval, including application assessment, technical capability assessment, prototype testing, and approval procedures. It also covers charges for various activities, validity of approvals, and requirements for new items or extensions. The Standing Committee on Small Track Machines examines cases and makes recommendations to the Railway Board for final approval.
Rdso vendor registration guideline

This document provides guidelines for vendor development and approval for small track machines and tools by the Research Designs and Standards Organisation (RDSO) of the Government of India Ministry of Railways.
It defines key terms, outlines the policy and process for vendor registration and approval, including application assessment, technical capability assessment, prototype testing, and approval procedures. It also covers charges for various activities, validity of approvals, and requirements for new items or extensions. The Standing Committee on Small Track Machines examines cases and makes recommendations to the Railway Board for final approval.
Medical Device Administration In China

This document discusses medical device administration in China. It outlines the competent authorities that regulate medical devices, including the SFDA and Center for Medical Device Evaluation. It also describes the administration activities like registration, production, sales, and post-marketing surveillance. The document then summarizes the laws and regulations regarding medical devices and classifies medical devices into three categories based on risk. It provides details on administration of production, sales, and registration of medical devices in China.
CE Marking , FDA Approval and Associated Regulations for Wellness

This document discusses key regulatory considerations for medical device approval in major markets. It provides an overview of regulatory strategies and approval processes in the US, EU, Canada and other countries. Major topics covered include device classification systems, premarket approval requirements, quality management standards, clinical data requirements and post-market obligations. Common mistakes made by companies and the importance of developing a comprehensive regulatory strategy early on are also addressed.
Strategies for Device Approval in China, India, South Korea and Australia

This session will describe the orthopaedic device regulatory and registration requirements in Asia Pacific markets. Regulatory steps and strategies will be presented for each of these countries. The discussion will also cover ways to gain regulatory information about competitors already selling in these markets. Attendees will leave the session with an understanding of timelines, costs and complexity for approval.
Cost records and audit rules 2014

This document provides details on cost accounting records and audit rules issued by the government of India in 2014. It summarizes the key provisions around the application of cost records and cost audits for different types of companies based on industry, turnover, and net worth. It also outlines the various cost heads that must be captured in cost records like material, salaries, utilities, depreciation, research and development, and others.
Cost records and audit rules 2014

This document provides details on cost records and audit rules issued by the government of India in 2014. It summarizes the key provisions around the application of cost records and cost audit for different types of companies based on industry, turnover, net worth, and products. It also outlines the various cost heads that must be captured in cost records like material, salaries, utilities, service department expenses, repairs and maintenance, fixed assets, overheads, R&D, quality control, work in progress, and more.
Medical Devices Rules 2017 Implementation

Medical Devices Rules are now enforced for all medical devices. It is important to know about the MDR 2017 and how it affects the Manufacturers, Importers and Distributors of Medical Devices and status of implementation.
Scheme for Notifying Examiner of Electronic Evidence Under section 79A of the...

Scheme for Notifying Examiner of Electronic Evidence Under section 79A of the Information Technology Act 2000
Equipment qualification

This document provides an overview of equipment qualification terminology and processes. It discusses conducting a risk assessment to determine validation requirements and maintaining qualified equipment. The key points covered include defining user requirements, performing installation qualification to verify proper installation, and operational qualification to confirm the equipment operates as intended. Maintaining qualification involves ongoing change control and periodic review.
Equipment qualification

This document provides an overview of equipment qualification terminology and processes. It discusses conducting a risk assessment to determine validation requirements and maintaining qualified equipment. The key points covered include defining user requirements, performing installation qualification to verify proper installation, and operational qualification to confirm the equipment operates as intended. Maintaining qualification involves ongoing change control and periodic review.
SPECS-Guidelines-Presentation.pdf

Scheme for Promotion of Manufacturing of Electronic Components
and Semiconductors (SPECS)
Applicant: Private Limited Company, Public Limited Company, Sole Proprietorship,
Partnership, or Limited Liability Partnership registered in India.
2. Project / Unit: New business unit or expansion of capacity / modernization and / or
diversification of an existing unit.
3. Expansion of capacity / modernization and/ or diversification of an existing unit: An
increase in the value of fixed capital investment in plant, machinery, equipment, associated
utilities and technology, including for Research & Development (R&D) of an existing unit.
4. Approved Project / Unit: Project for which approval is issued by the Project Management
Agency (PMA), based on the recommendations of Executive Committee (EC) under MeitY
1. Expenditure incurred on plant, machinery, equipment and associated utilities:
• tools, dies, moulds, jigs, fixtures (including parts, accessories, components, and spares
thereof) of the same.
• expenditure on packaging, freight/ transport, insurance, and erection and
commissioning.
• The Associated utilities - captive power and effluent treatment plants, essential equipment
required in operations areas such as clean rooms, air curtains, temperature and air quality
control systems, compressed air, water & power supply and control systems, etc.
• The Associated utilities also include IT and ITES infrastructure related to manufacturing
including servers, software and ERP solutions.
• The total expenditure incurred on associated utilities - not exceeding 20% of the total
eligible capital expenditure for plant, machinery and equipment.
BIS REGISTRATION FOR WEBCAM (FINISHED PRODUCT) – IS 616 : 2017

This document provides guidelines for registering a webcam product with the Bureau of Indian Standards (BIS). It outlines the applicable BIS standard IS 616:2017, the compulsory registration scheme, certification authority as BIS, and relevant quality control order. The contents section lists the topics to be covered including BIS registration process, requirements, documentation, testing, and granting of the license. It also discusses inclusion of new models and license renewal. Overall, the document guides manufacturers through BIS registration for a webcam product in India.
ISO 17065 .pdf

Product certification or product qualification is the process of certifying that a certain
product has passed performance tests and quality assurance tests, and meets
qualification criteria stipulated in contracts, regulations, or specifications (sometimes
called "certification schemes" in the product certification industry).
Most product certification bodies (or product certifiers) are accredited to or aligned with
ISO/IEC 17065 Conformity assessment -- Requirements for bodies certifying products,
processes and services (previously ISO/IEC Guide 65:1996) an international standard
for ensuring competence in those organizations performing product, process and service
certifications. The organizations that perform this accreditation are called Accreditation
Bodies, and they themselves are assessed by international peers against the ISO 17011
standard. In India Product certification is being monitored by BUREAU OF INDIAN
STANDARDS.
Examples of some certification schemes include the Safety Equipment Institute for
protective headgear, the U.S. Federal Communications Commission (FCC)
Telecommunication Certification Body (TCB) program for radio communication devices,
the U.S. Environmental Protection Agency Energy Star program, the International
Commission on the Rules for the Approval of Electrical Equipment Product Safety
Certification Body Scheme (IEECE CB Scheme), MAS (Materials Analytical Services)
Certified Green IEQ program, and the Greenguard Environmental Institute Indoor Air
Quality program. Certification schemes are typically written to include both the
performance test methods that the product must be tested to, as well as the criteria that
the product must meet to become Certified.
EMA Qualification & Validation Requirements 

This presentation describes the principles of qualification and validation which are applicable to the facilities, equipment, utilities and processes used for the manufacture of medicinal products. It is a GMP requirement that manufacturer’s control the critical aspects of their particular operations through qualification and validation over the life cycle of the product and process. Any planned changes to the facilities, equipment, utilities and processes, which may affect the quality of the product, should be formally documented and the impact on the validated status or control strategy assessed.
Medical device regulations

In the USA, medical devices are regulated by the Food and Drug Administration (FDA) with an aim to ensure safety and effectiveness of the devices. The Center for Devices and Radiological Health (CDRH) is an FDA component and looks after this program.
Cost records and audit rules 2014

This document outlines the key details of the Cost Records and Audit Rules 2014 issued by the Government of India. It provides information on the application and implementation of cost record rules for different industries. Some of the key points covered include the threshold limits for maintenance of cost records based on net worth and turnover, details to be captured for material, salaries, utilities, service departments and other overheads, treatment of by-products and joint products, and allocation of expenses related to R&D, exports and pollution control. The document also describes the format and details to be reported in CRA-1 form for cost records.
Equipment Qualification

Equipment used in pharmaceuticals dosage form manufacturing need to observe continuous qualification to monitor its performance and Concept of URS ,DQ, IQ,OQ,PQ,MQ...
Key Differences between Medical Device User Fees and Establishment Registrati...

This document compares medical device user fees and establishment registration and listing requirements with the FDA. Medical device user fees are paid by applicants submitting applications for product review, such as 510(k)s or PMAs. Establishment registration fees are paid annually by companies involved in medical device production and distribution. Key differences include what entities must pay each fee, annual versus multi-year fee structures, exemptions for small businesses from some user fees, and different processes for payment and submission of registration information. Understanding the distinction between these fee types is important for medical device companies to comply with FDA regulatory requirements.
Similar to South Korea medical device approval chart - Emergo (20)
CE Marking , FDA Approval and Associated Regulations for Wellness

CE Marking , FDA Approval and Associated Regulations for Wellness
Strategies for Device Approval in China, India, South Korea and Australia

Strategies for Device Approval in China, India, South Korea and Australia
Scheme for Notifying Examiner of Electronic Evidence Under section 79A of the...

Scheme for Notifying Examiner of Electronic Evidence Under section 79A of the...
BIS REGISTRATION FOR WEBCAM (FINISHED PRODUCT) – IS 616 : 2017

BIS REGISTRATION FOR WEBCAM (FINISHED PRODUCT) – IS 616 : 2017
Key Differences between Medical Device User Fees and Establishment Registrati...

Key Differences between Medical Device User Fees and Establishment Registrati...
Recently uploaded
CCSN_June_06 2024_jones. Cancer Rehabpptx

About this webinar: This talk will introduce what cancer rehabilitation is, where it fits into the cancer trajectory, and who can benefit from it. In addition, the current landscape of cancer rehabilitation in Canada will be discussed and the need for advocacy to increase access to this essential component of cancer care.
How Effective is Homeopathic Medicine for Anxiety and Stress Relief.pdf

Have you ever wondered how effective is homeopathic medicine for anxiety and stress relief? Let’s dive into this fascinating topic.
KEY Points of Leicester travel clinic In London doc.docx

In order to protect visitors' safety and wellbeing, Travel Clinic Leicester offers a wide range of travel-related health treatments, including individualized counseling and vaccines. Our team of medical experts specializes in getting people ready for international travel, with a particular emphasis on vaccines and health consultations to prevent travel-related illnesses. We provide a range of travel-related services, such as health concerns unique to a trip, prevention of malaria, and travel-related medical supplies. Our clinic is dedicated to providing top-notch care, keeping abreast of the most recent recommendations for vaccinations and travel health precautions. The goal of Travel Clinic Leicester is to keep you safe and well-rested no matter what kind of travel you choose—business, pleasure, or adventure.
Hypotension and role of physiotherapy in it

This particular slides consist of- what is hypotension,what are it's causes and it's effect on body, risk factors, symptoms,complications, diagnosis and role of physiotherapy in it.
This slide is very helpful for physiotherapy students and also for other medical and healthcare students.
Here is the summary of hypotension:
Hypotension, or low blood pressure, is when the pressure of blood circulating in the body is lower than normal or expected. It's only a problem if it negatively impacts the body and causes symptoms. Normal blood pressure is usually between 90/60 mmHg and 120/80 mmHg, but pressures below 90/60 are generally considered hypotensive.
Innovative Minds France's Most Impactful Healthcare Leaders.pdf

This edition features a handful of Innovative Minds: France's Most Impactful Healthcare Leaders that are leading us into a better future.
Can Allopathy and Homeopathy Be Used Together in India.pdf

This article explores the potential for combining allopathy and homeopathy in India, examining the benefits, challenges, and the emerging field of integrative medicine.
CANSA support - Caring for Cancer Patients' Caregivers

International Cancer Survivors Day is celebrated during June, placing the spotlight not only on cancer survivors, but also their caregivers.
CANSA has compiled a list of tips and guidelines of support:
https://cansa.org.za/who-cares-for-cancer-patients-caregivers/
Pneumothorax and role of Physiotherapy in it.

This particular slides consist of- what is Pneumothorax,what are it's causes and it's effect on body, risk factors, symptoms,complications, diagnosis and role of physiotherapy in it.
This slide is very helpful for physiotherapy students and also for other medical and healthcare students.
Here is a summary of Pneumothorax:
Pneumothorax, also known as a collapsed lung, is a condition that occurs when air leaks into the space between the lung and chest wall. This air buildup puts pressure on the lung, preventing it from expanding fully when you breathe. A pneumothorax can cause a complete or partial collapse of the lung.
LGBTQ+ Adults: Unique Opportunities and Inclusive Approaches to Care

This webinar helps clinicians understand the unique healthcare needs of the LGBTQ+ community, primarily in relation to end-of-life care. Topics include social and cultural background and challenges, healthcare disparities, advanced care planning, and strategies for reaching the community and improving quality of care.
Under Pressure : Kenneth Kruk's Strategy

Kenneth Kruk's story of transforming challenges into opportunities by leading successful medical record transitions and bridging scientific knowledge gaps during COVID-19.
Unlocking the Secrets to Safe Patient Handling.pdf

Furthermore, the time constraints and workload in healthcare settings can make it challenging for caregivers to prioritise safe patient handling Australia practices, leading to shortcuts and increased risks.
Michigan HealthTech Market Map 2024 with Policy Makers, Academic Innovation C...

Michigan HealthTech Market Map 2024. Includes 7 categories: Policy Makers, Academic Innovation Centers, Digital Health Providers, Healthcare Providers, Payers / Insurance, Device Companies, Life Science Companies, Innovation Accelerators. Developed by the Michigan-Israel Business Accelerator
NEEDLE STICK INJURY - JOURNAL CLUB PRESENTATION - DR SHAMIN EABENSON

NEEDLE STICK INJURY - JOURNAL CLUB PRESENTATION - DR SHAMIN EABENSON
Champions of Health Spotlight On Leaders Shaping Germany's Healthcare.pdf

This edition features a handful of Champions of Health: Spotlight On Leaders Shaping Germany's Healthcare that are leading us into a better future.
The Power of Superfoods and Exercise.pdf

Healthy Eating Habits:
Understanding Nutrition Labels: Teaches how to read and interpret food labels, focusing on serving sizes, calorie intake, and nutrients to limit or include.
Tips for Healthy Eating: Offers practical advice such as incorporating a variety of foods, practicing moderation, staying hydrated, and eating mindfully.
Benefits of Regular Exercise:
Physical Benefits: Discusses how exercise aids in weight management, muscle and bone health, cardiovascular health, and flexibility.
Mental Benefits: Explains the psychological advantages, including stress reduction, improved mood, and better sleep.
Tips for Staying Active:
Encourages consistency, variety in exercises, setting realistic goals, and finding enjoyable activities to maintain motivation.
Maintaining a Balanced Lifestyle:
Integrating Nutrition and Exercise: Suggests meal planning and incorporating physical activity into daily routines.
Monitoring Progress: Recommends tracking food intake and exercise, regular health check-ups, and provides tips for achieving balance, such as getting sufficient sleep, managing stress, and staying socially active.
Feeding plate for a newborn with Cleft Palate.pptx

A feeding plate is a prosthetic device used for newborns with a cleft palate to assist in feeding and improve nutrition intake. From a prosthodontic perspective, this plate acts as a barrier between the oral and nasal cavities, facilitating effective sucking and swallowing by providing a more normal anatomical structure. It helps to prevent milk from entering the nasal passage, thereby reducing the risk of aspiration and enhancing the infant's ability to feed efficiently. The feeding plate also aids in the development of the oral muscles and can contribute to better growth and weight gain. Its custom fabrication and proper fitting by a prosthodontist are crucial for ensuring comfort and functionality, as well as for minimizing potential complications. Early intervention with a feeding plate can significantly improve the quality of life for both the infant and the parents.
TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardso...

TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardso...rightmanforbloodline
TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardson, Verified Chapters 1 - 18, Complete Newest Version
TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardson, Verified Chapters 1 - 18, Complete Newest Version
TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardson, Verified Chapters 1 - 18, Complete Newest VersionTop Rated Massage Center In Ajman Chandrima Spa

The best massage spa Ajman is Chandrima Spa Ajman, which was founded in 2023 and is exclusively for men 24 hours a day. As of right now, our parent firm has been providing massage services to over 50,000+ clients in Ajman for the past 10 years. It has about 8+ branches. This demonstrates that Chandrima Spa Ajman is among the most reasonably priced spas in Ajman and the ideal place to unwind and rejuvenate. We provide a wide range of Spa massage treatments, including Indian, Pakistani, Kerala, Malayali, and body-to-body massages. Numerous massage techniques are available, including deep tissue, Swedish, Thai, Russian, and hot stone massages. Our massage therapists produce genuinely unique treatments that generate a revitalized sense of inner serenely by fusing modern techniques, the cleanest natural substances, and traditional holistic therapists.
Recently uploaded (20)
How Effective is Homeopathic Medicine for Anxiety and Stress Relief.pdf

How Effective is Homeopathic Medicine for Anxiety and Stress Relief.pdf
KEY Points of Leicester travel clinic In London doc.docx

KEY Points of Leicester travel clinic In London doc.docx
Anxiety, Trauma and Stressor Related Disorder.pptx

Anxiety, Trauma and Stressor Related Disorder.pptx
Innovative Minds France's Most Impactful Healthcare Leaders.pdf

Innovative Minds France's Most Impactful Healthcare Leaders.pdf
Can Allopathy and Homeopathy Be Used Together in India.pdf

Can Allopathy and Homeopathy Be Used Together in India.pdf
CANSA support - Caring for Cancer Patients' Caregivers

CANSA support - Caring for Cancer Patients' Caregivers
LGBTQ+ Adults: Unique Opportunities and Inclusive Approaches to Care

LGBTQ+ Adults: Unique Opportunities and Inclusive Approaches to Care
Unlocking the Secrets to Safe Patient Handling.pdf

Unlocking the Secrets to Safe Patient Handling.pdf
Michigan HealthTech Market Map 2024 with Policy Makers, Academic Innovation C...

Michigan HealthTech Market Map 2024 with Policy Makers, Academic Innovation C...
NEEDLE STICK INJURY - JOURNAL CLUB PRESENTATION - DR SHAMIN EABENSON

NEEDLE STICK INJURY - JOURNAL CLUB PRESENTATION - DR SHAMIN EABENSON
Champions of Health Spotlight On Leaders Shaping Germany's Healthcare.pdf

Champions of Health Spotlight On Leaders Shaping Germany's Healthcare.pdf
Feeding plate for a newborn with Cleft Palate.pptx

Feeding plate for a newborn with Cleft Palate.pptx
TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardso...

TEST BANK For Accounting Information Systems, 3rd Edition by Vernon Richardso...
South Korea medical device approval chart - Emergo
- 1. The regulatory process for medical devices KRSouth Korea * If your device (all classes) is new or does not have a predicate in the South Korean market, then the MFDS will require a Clinical Data Review, formerly Safety and Efficacy Review (SER). Manufacturers will be required to submit Clinical Data with their application and the registration approval timeline and fees will increase accordingly. ** There are approximately 350 Class II devices that MFDS considers “Special Equivalent,” which require a less robust application, i.e. no Technical File, as well as an expedited review by a Third Party Reviewer. *** Prior testing done in accordance with ISO or other international standards will satisfy some testing requirements; however, most manufacturers have to conduct additional testing, including performance testing, to Korea specific product standards. This is a simplified overview of the process. The MFDS may choose to audit your submission and request more documents, which will add time to your approval. © 2015 Emergo – You are welcome to publish this chart on your website, or copy it for use in presentations or other materials if it is not cropped in any way. Have comments or suggestions about the content of this chart? Email us at marketing@emergogroup.com. Chart updated 03/2015. 5040-0315 EmergoGroup.com/south-korea Determine classification of your device based on the device database, regulations provided by South Korea’s Ministry of Food and Drug Safety (MFDS), as well as predicate devices in the South Korean market. If no predicate exists, the device will follow the Clinical Data Review route.* Class I Class II** Class III Class IV Ifyou do not have a local office in South Korea,you must appoint a Korea License Holder based in the countryto manageyour medical device registration with the MFDS. Prepare Pre-Market Notification including basic device information. Prepare registration application in Korean, including technical file, test reports and KGMP certificate. Prepare registration application in Korean, including technical file, test reports and KGMP certificate. Class IV devices require an additional technical file in STED format. Send your device to Korea for testing OR prepare existing equivalent Korea testing results. *** Appoint a qualified importer/distributor to bring your device into South Korea. You are now authorized to begin marketing your device. KLH submits Pre-Market Notification application to the MFDS database. Pay application fees. KLHsubmitsKoreanregistrationdossierto acertifiedThirdPartyReviewer. Application includestechnicalfile,testreports,and KGMPCertificate. AftersuccessfulThird PartyReview,theMFDSwillissuethefinal approval.Payapplicationfees. KLH submits Korean registration dossier to the MFDS for review. Dossier includes the Technical File plus STED Technical File (Class IV), test reports, and KGMP certificate. Pay application fees. Obtain Korea Good Manufacturing Practices (KGMP) certificationthrough an onsite audit. Class II device manufacturing facilities are usuallyaudited byaThird PartyAuditor. KGMPcertification must be renewed every3years. Obtain Korea Good Manufacturing Practices (KGMP) certification through an onsite audit. Class III and IV device manufacturing facilities are audited by a Third Party Auditor and the MFDS. KGMPcertification must be renewed every3years. Class I devices do not require approval, only Notification to the MFDS. The MFDS will publish the device on their website. Notifications do not expire. Full application review conducted. Pre-Market Approval license is issued by the MFDS and published on their website. Registrations do not expire.
- 2. The regulatory process for medical devices EmergoGroup.com/south-korea This is a simplified overview of the process. South Korea’s Ministry of Food and Drug Safety (MFDS) may choose to audit your submission and request more documents, which will add time to your approval. NOTE 1: The time frames shown above are typical for the majority of medical device submissions but assume that your device does not contain animal tissue, medicinal substances or employ entirely novel technology. Your length of approval will depend on the quality and completeness of your technical documentation and how much time you take to address additional information requests from authorities after submission. YOUR SUBMISSION(S) MAY TAKE MORE TIME THAN WHAT IS SHOWN ABOVE. NOTE 2: The device license does not expire, but is subject to reevaluation. Periodically the MFDS will select devices codes to reevaluate as a whole. Typically this does not apply to products that were recently approved under that selected device code, as this process is intended to ensure older products meet the current regulatory requirements. Manufacturers will need to submit documents for reevaluation; documents include but are not limited to: updated vigilance, updated QMS documents, updated approvals from other markets. Announcements of device codes up for reevaluation typically happen in April or May, and manufacturers must submit before the end of the year. Failure to comply with the reevaluation will cause the device license to be revoked. NOTE 3: The device notification (Class I) and license (Class II-IV) does not expire, so no renewal is required. NOTE 4: Our rating of the complexity of the registration process is based on our experience and the opinion of nearly 1,000 QA/RA professionals worldwide who were asked to rate the difficulty of registering a device in each country. The European CE Marking process is considered the mid-point to which all other markets are compared. NOTE 5: Low = Less than US$5000; Midpoint = US$15000-$30000: High = More than US$50000. Overall cost includes registration application fees, product testing, in-country representation, submission preparation consulting and translation of registration documents but not IFU. Costs assume you already have approval for your device in the United States, Europe, Canada or Japan. Does not include cost of implementing or auditing a quality management system, if applicable. * If your device (all classes) is new or does not have a predicate in the South Korean market, then the MFDS will require a Clinical Data Review, formerly Safety and Efficacy Review (SER). Manufacturers will be required to submit Clinical Data with their application and the registration approval timeline and fees will increase accordingly. †† There are approximately 350 Class II devices that MFDS considers “Special Equivalent,” which require a less robust application, i.e. no Technical File, as well as an expedited review by a Third Party Reviewer. © 2015 Emergo – You are welcome to publish this chart on your website, or copy it for use in presentations or other materials if it is not cropped in any way. Have comments or suggestions about the content of this chart? Email us at marketing@emergogroup.com. Chart updated 03/2015. Device classification in South Korea How long you should expect to wait after submission until approval is granted. (See note 1) Validity period for device registrations. (See note 2) Registration renewal should be started this far in advance. (See note 3) Complexity of the registration process for this classification. (See note 4) Overall cost of gaining regulatory approval. (See note 5) CLASS I 1 month Does not expire Not applicable Simple Complex Low High CLASS II*† 4-6 months Does not expire Not applicable Simple Complex Low High CLASS III* 6-10 months Does not expire Not applicable Simple Complex Low High CLASS IV* 6-10 months Does not expire Not applicable Simple Complex Low High KRSouth Korea
