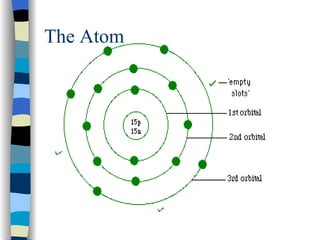
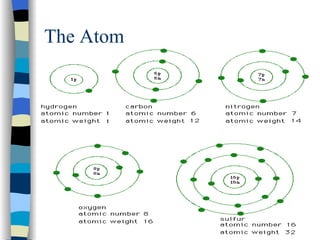
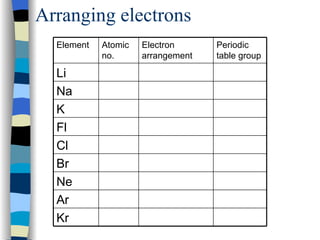
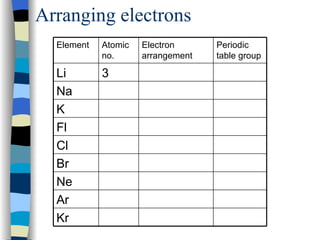
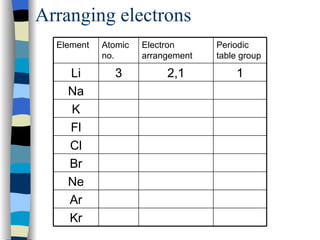
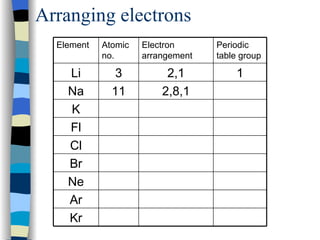
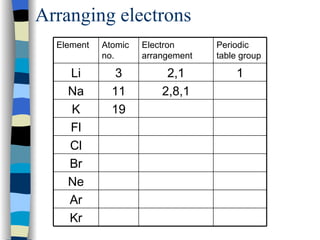
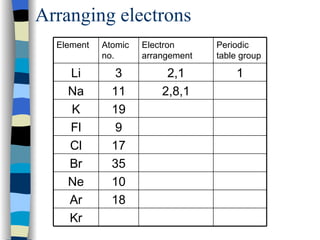
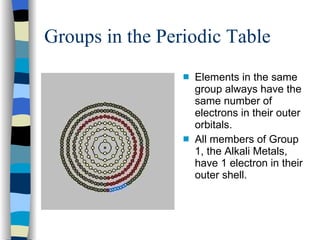
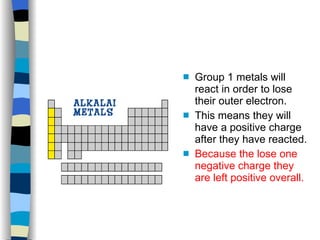
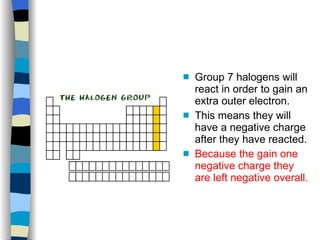
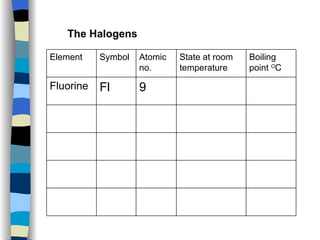
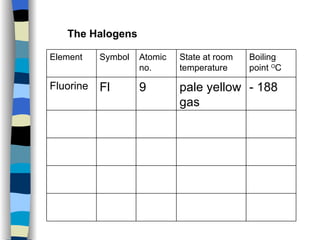
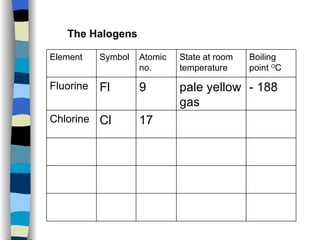
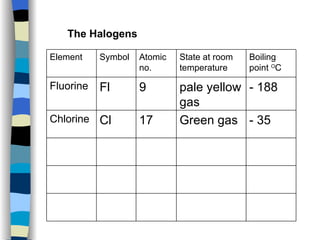
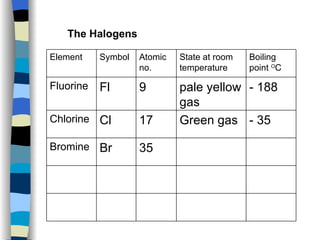
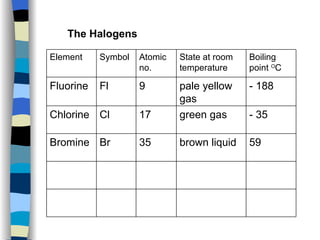
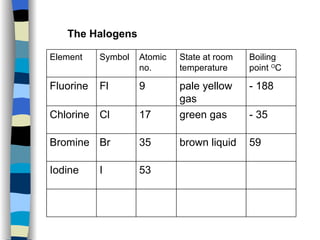
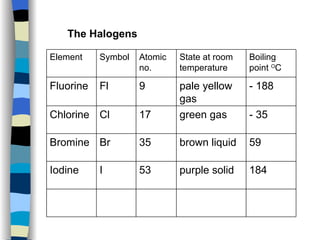
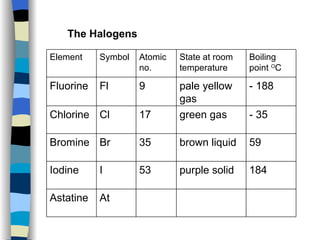
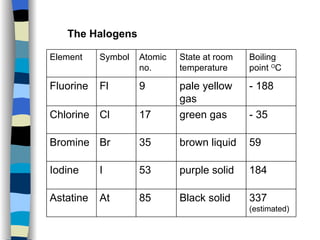
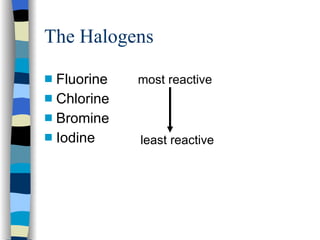
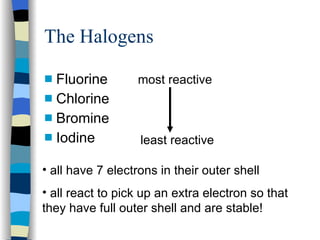
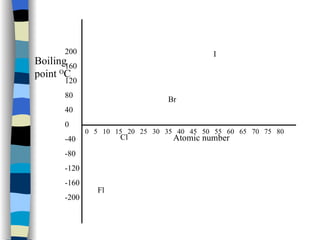
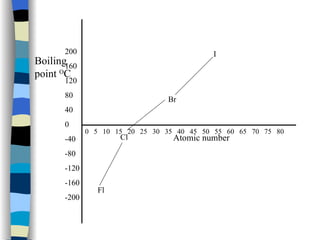
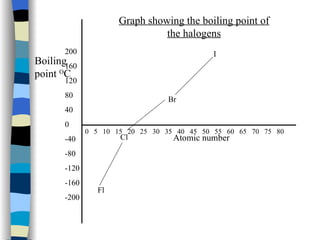
The document discusses the properties of the halogen elements fluorine, chlorine, bromine, iodine, and astatine. It explains that they are in group 7 of the periodic table and have 7 electrons in their outer shell. It wants to gain an extra electron to achieve a stable full outer shell. It provides details about the physical states, colors, reactivities and boiling points of the halogens. Fluorine is the most reactive and astatine is the least stable and rarest of the group.