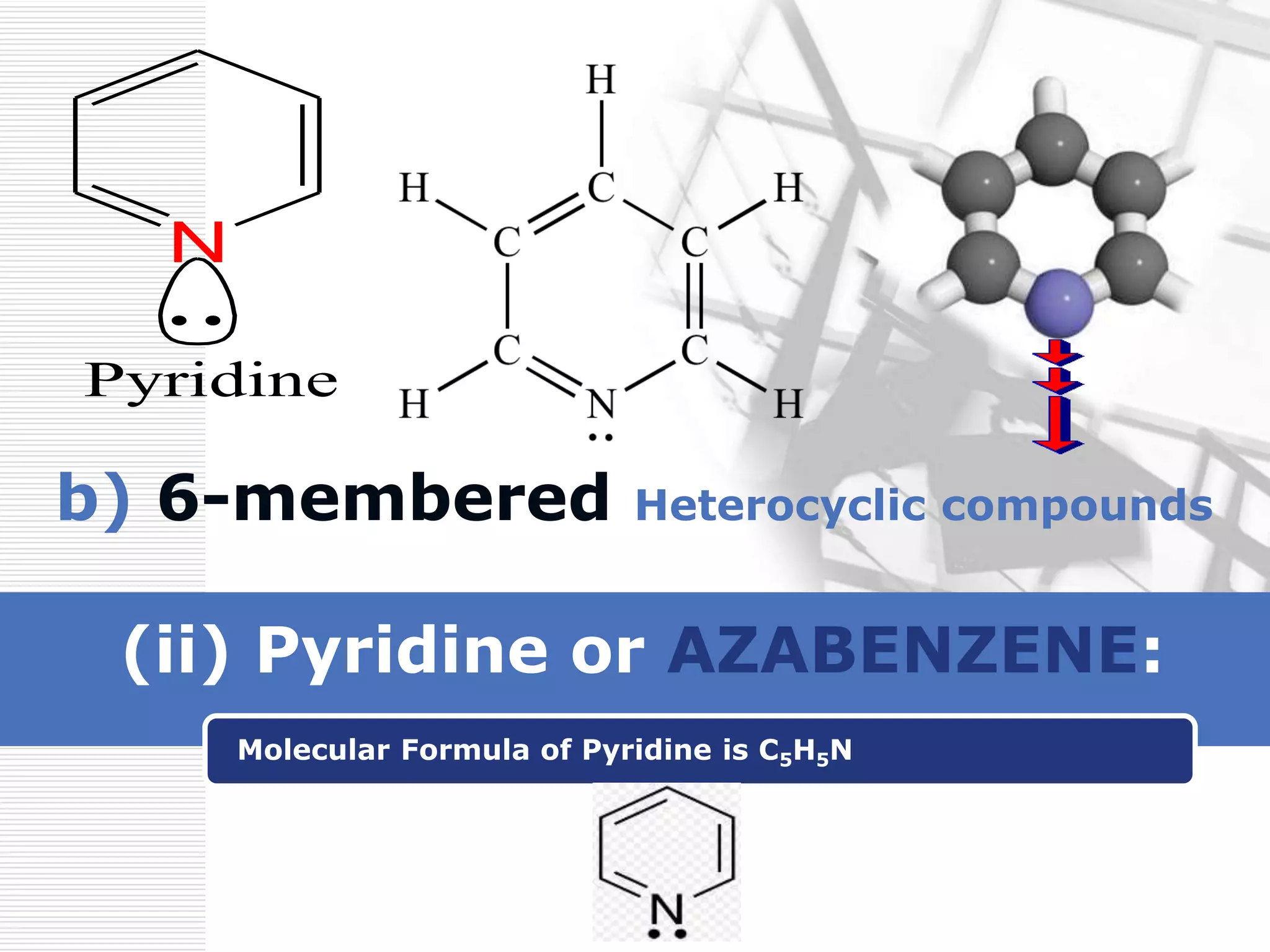
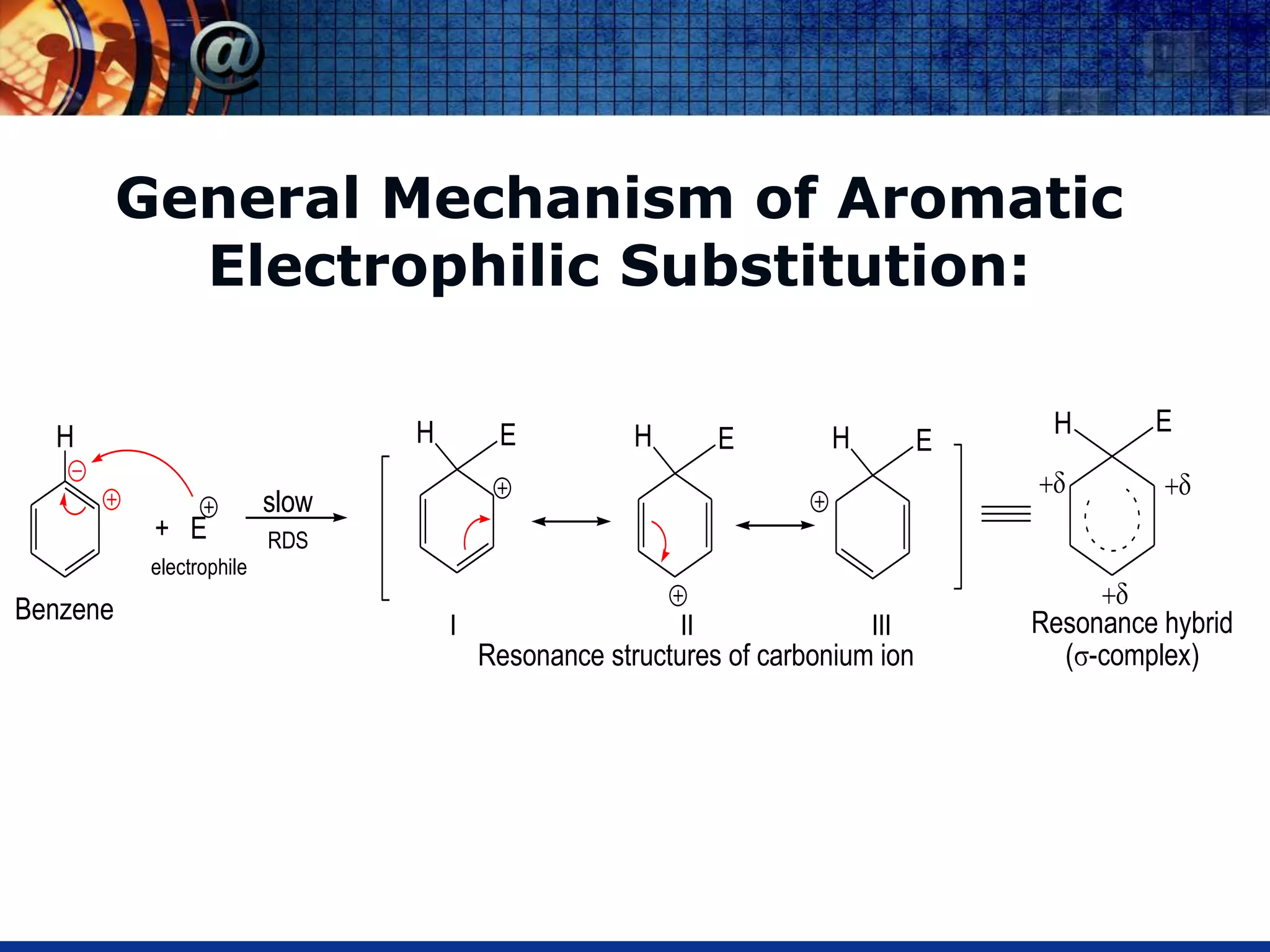
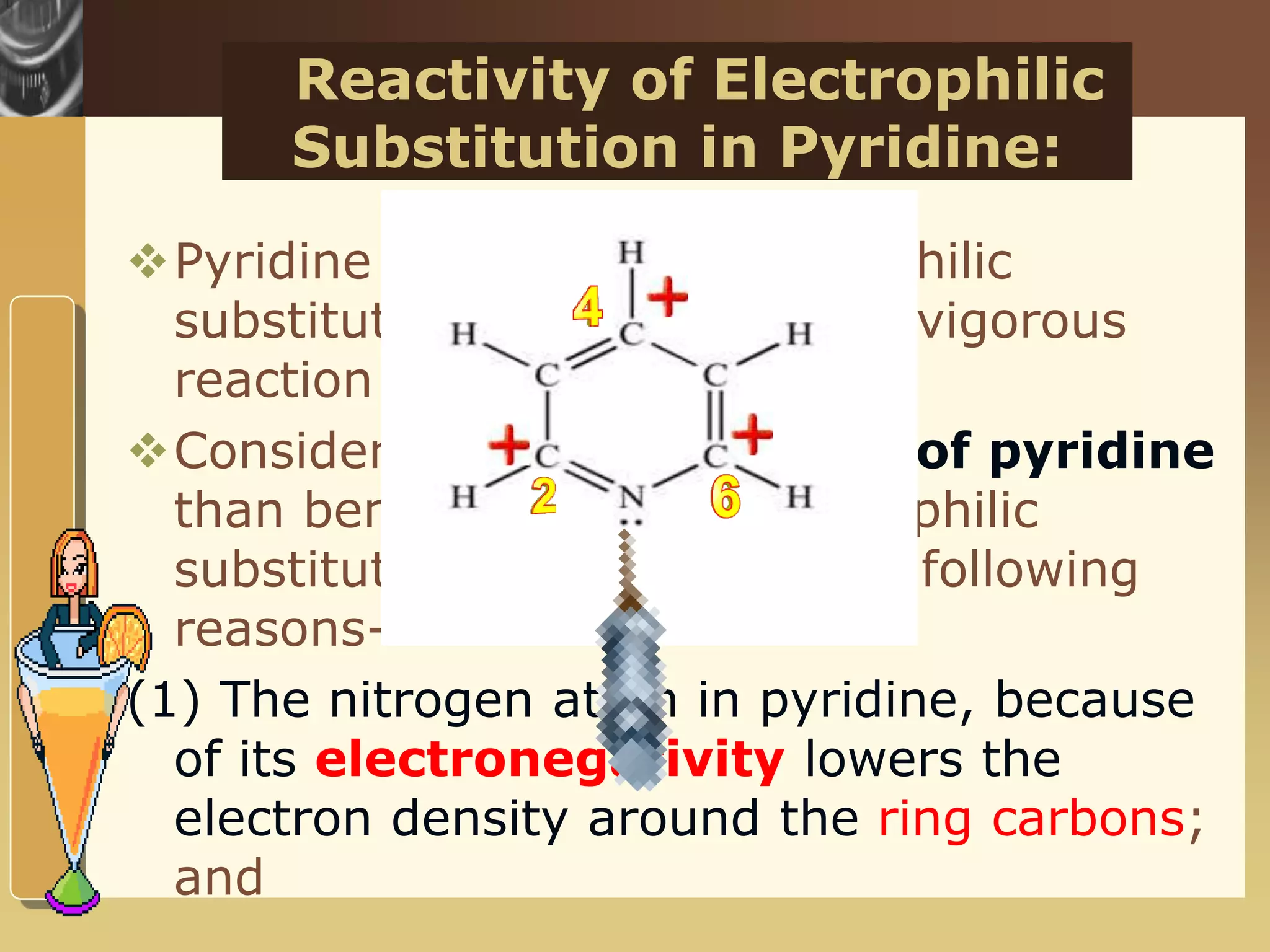
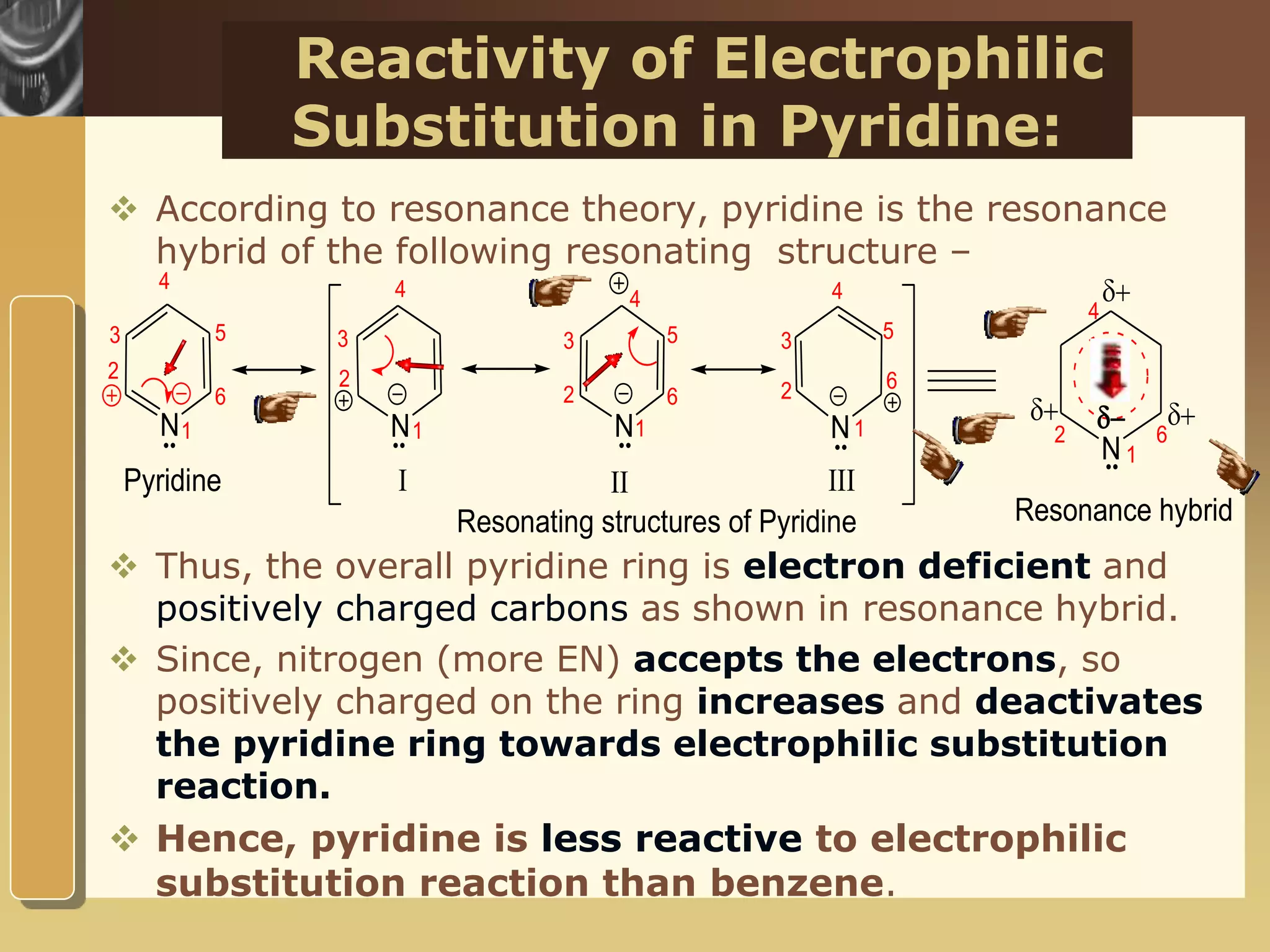
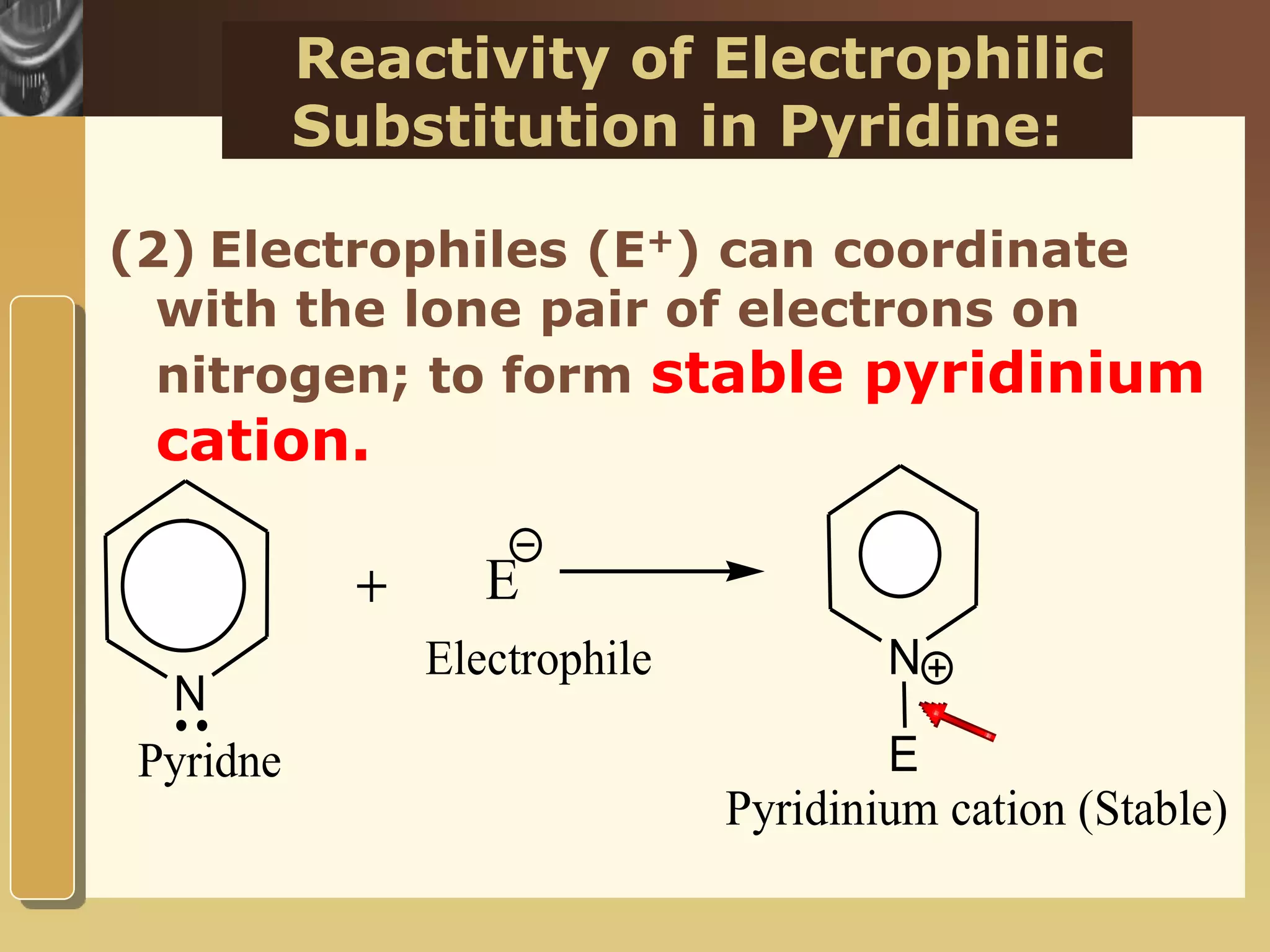
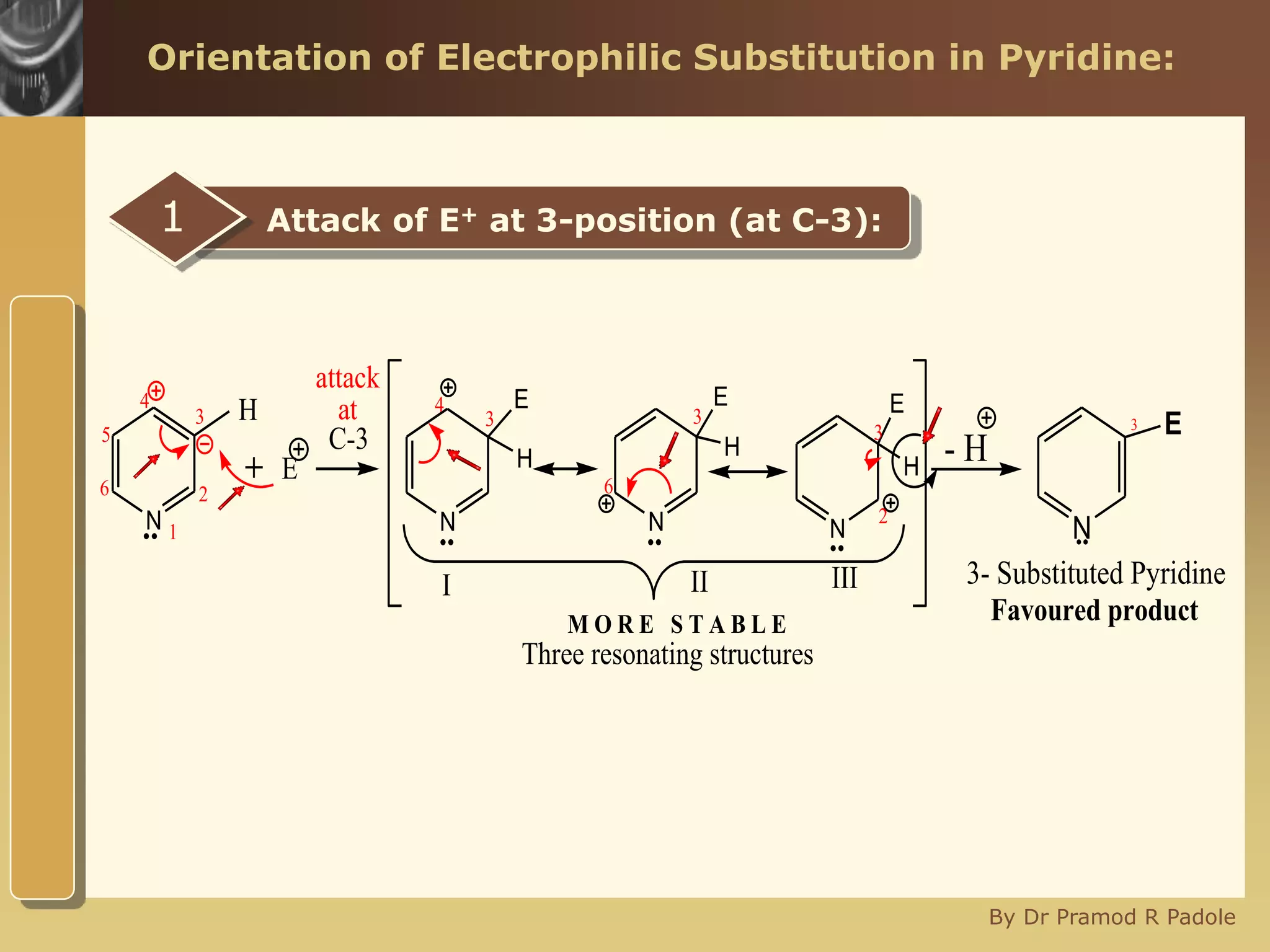
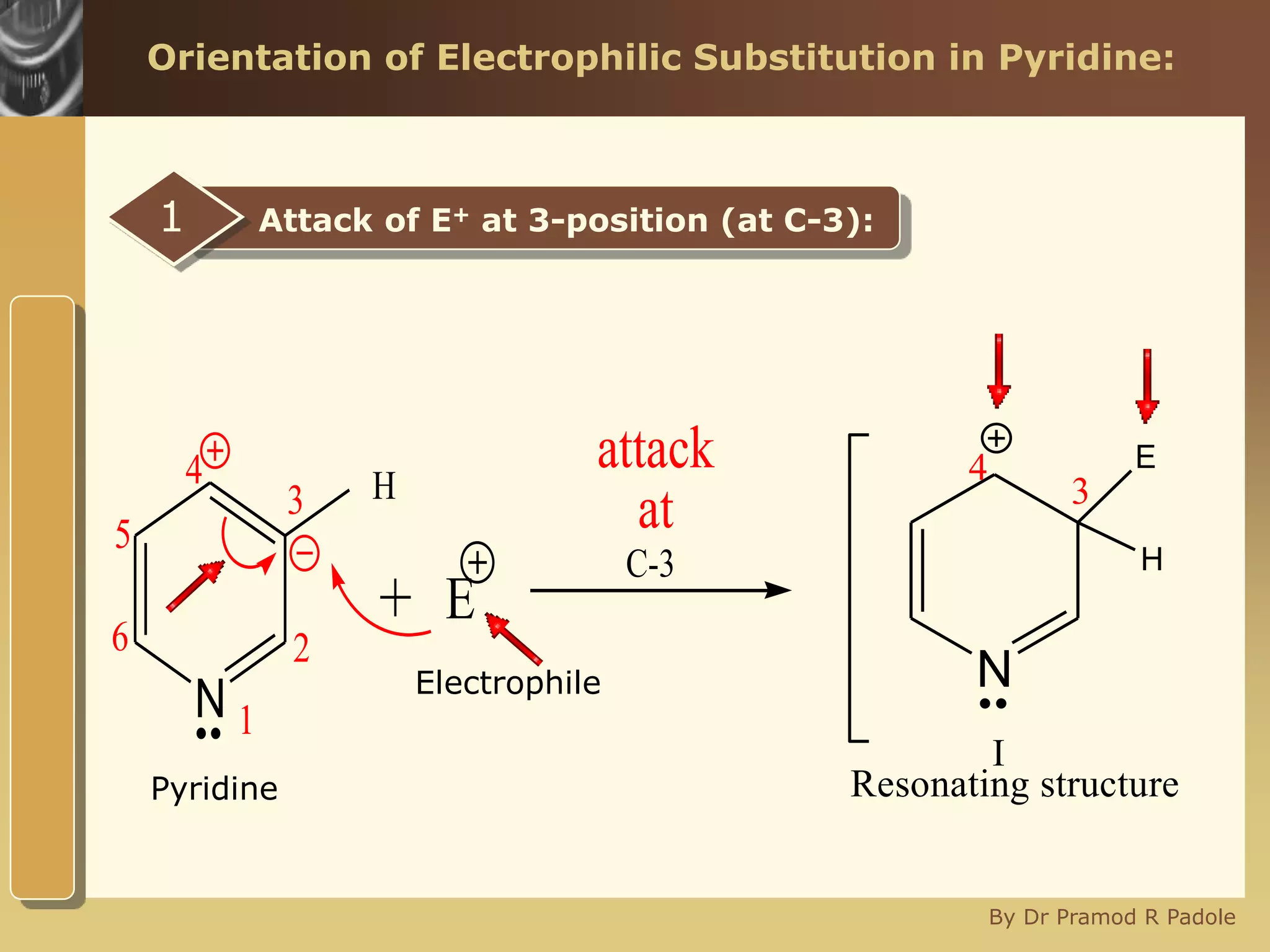
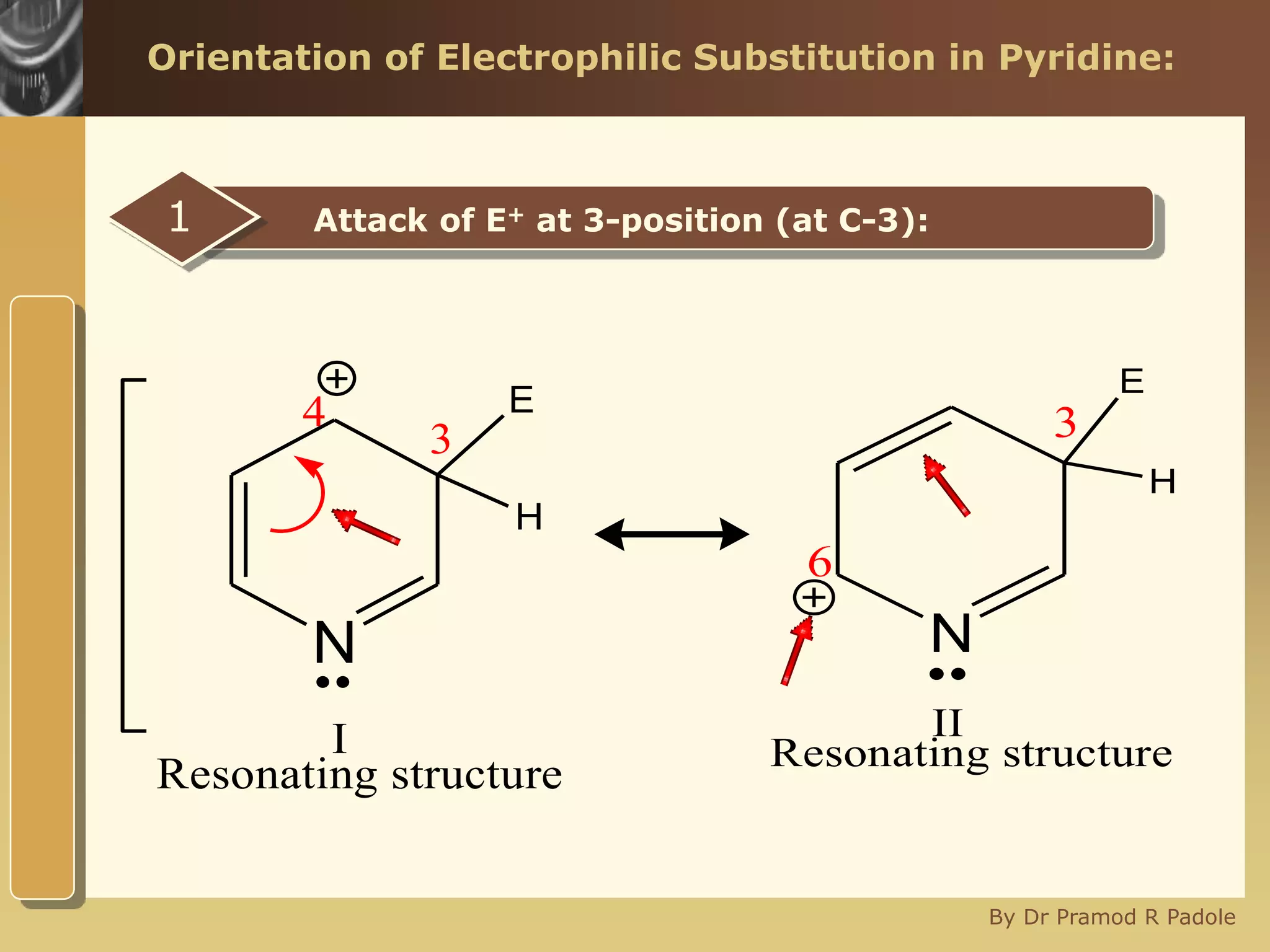
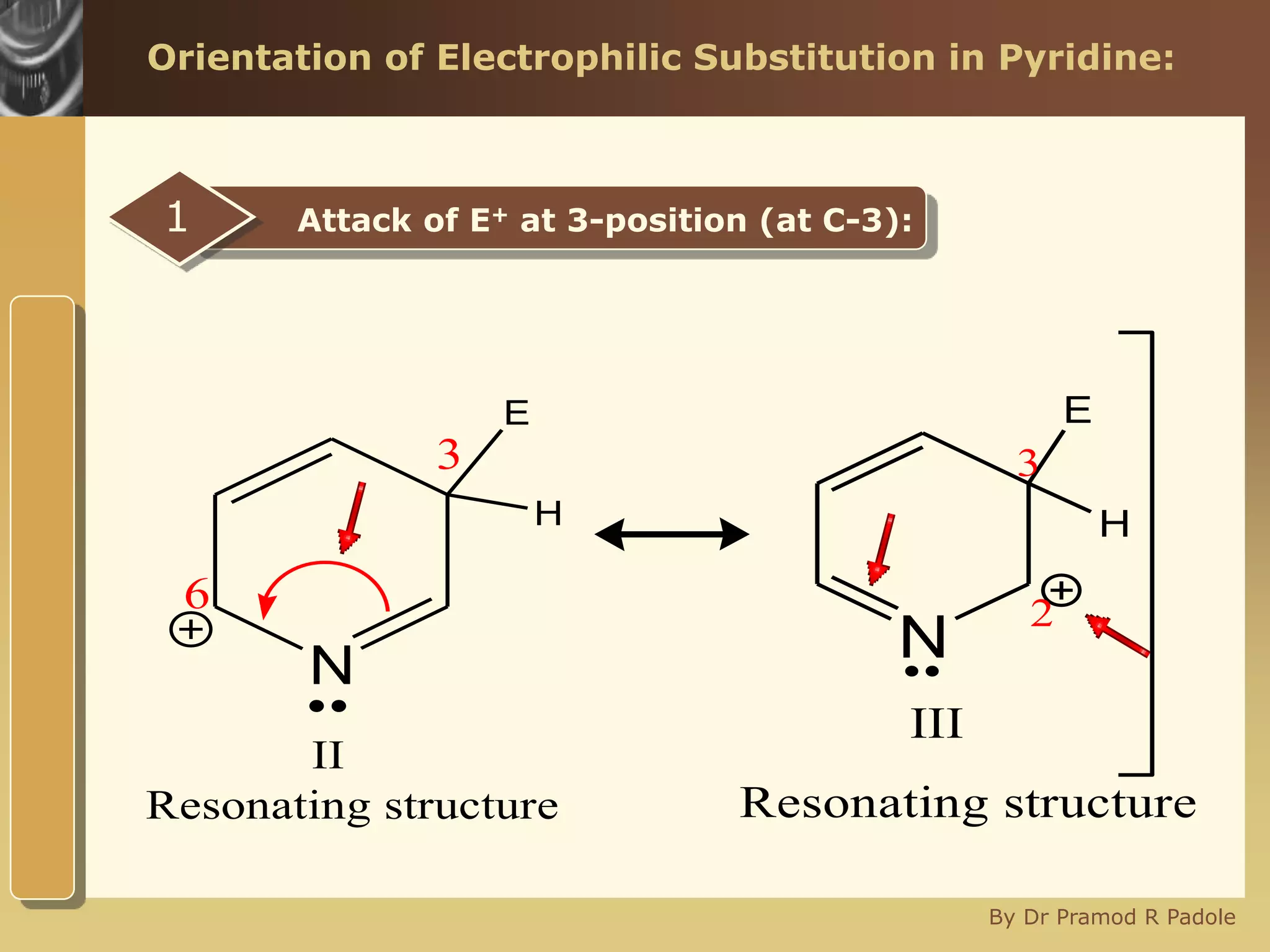
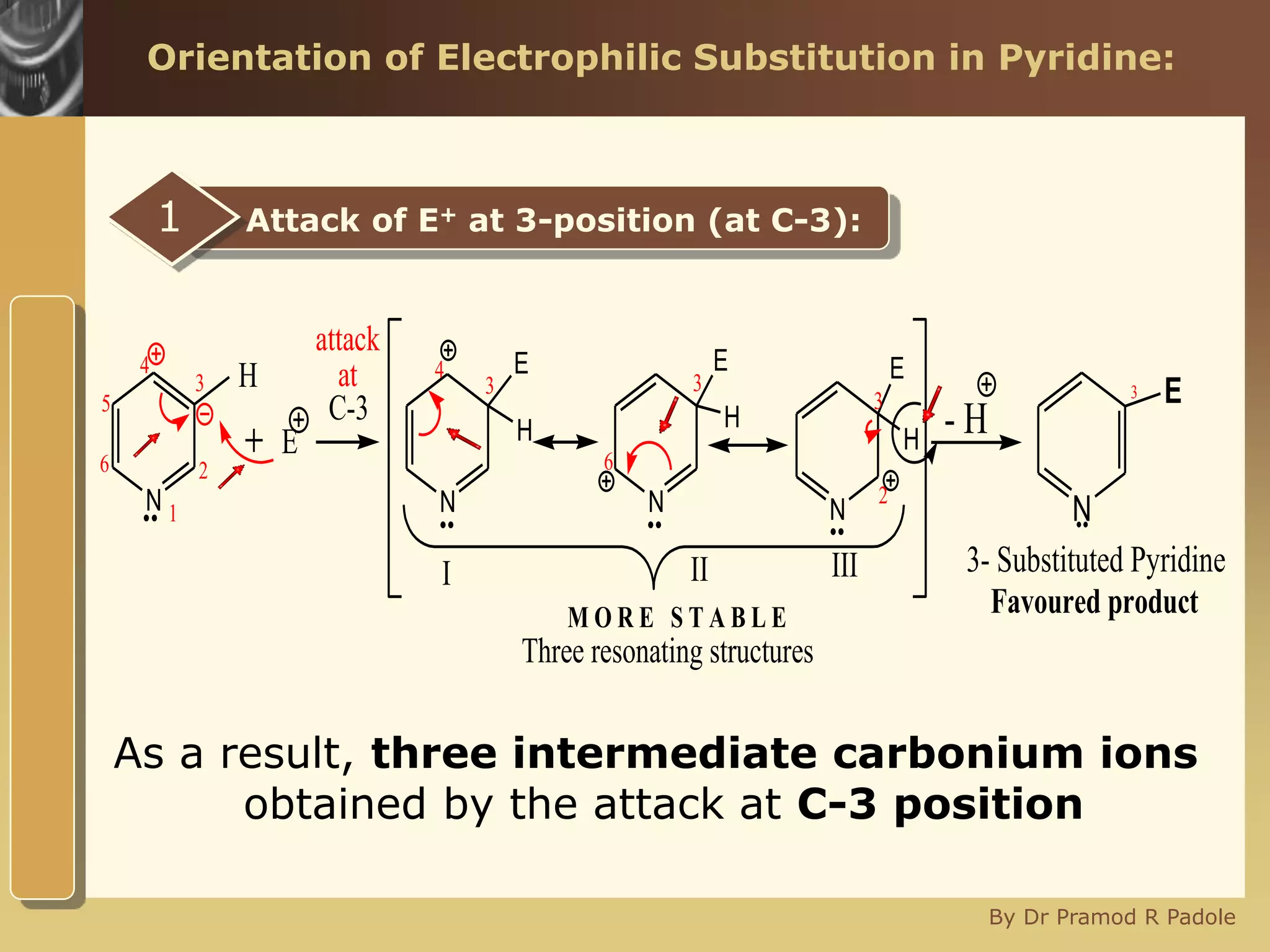
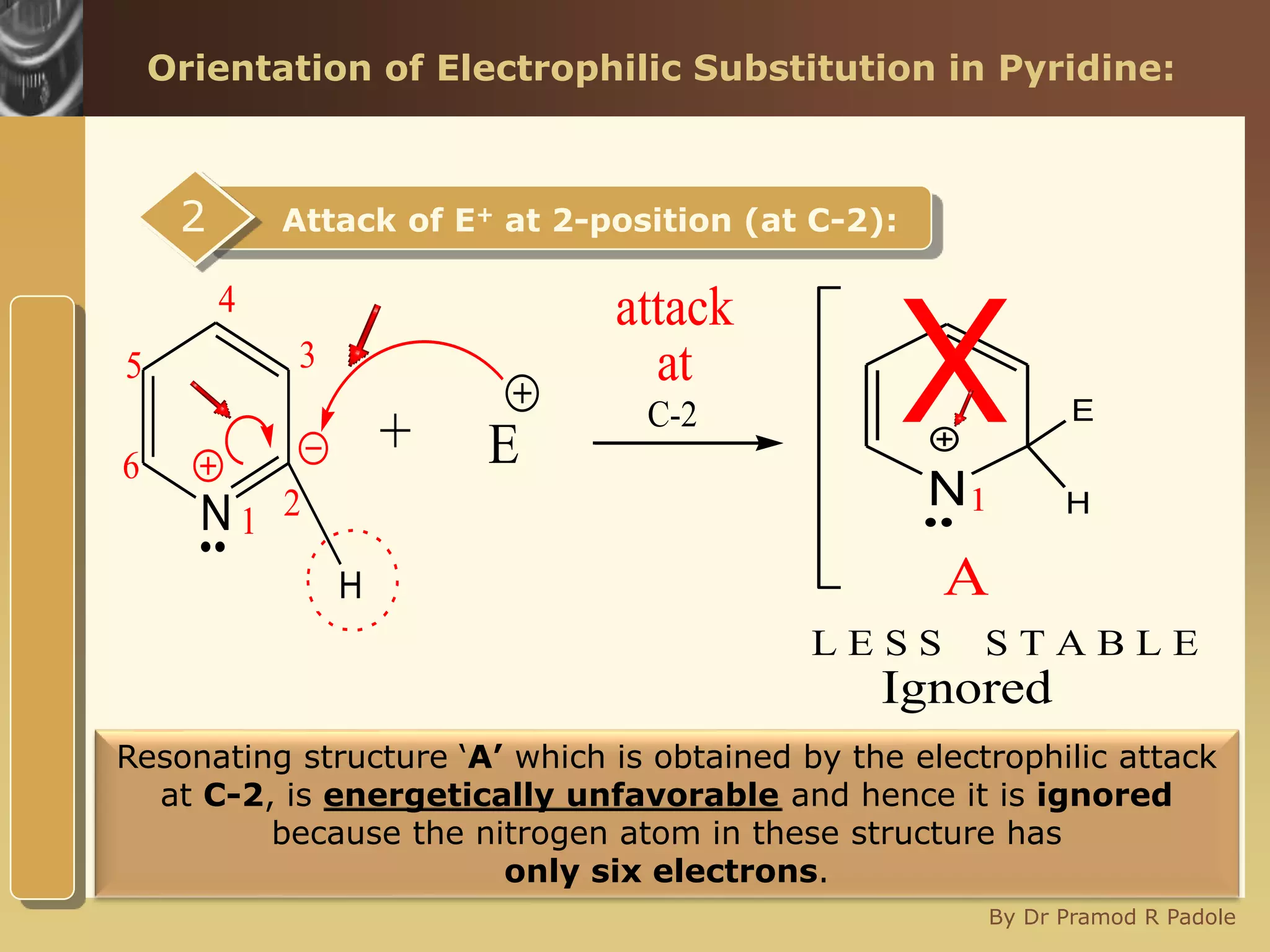
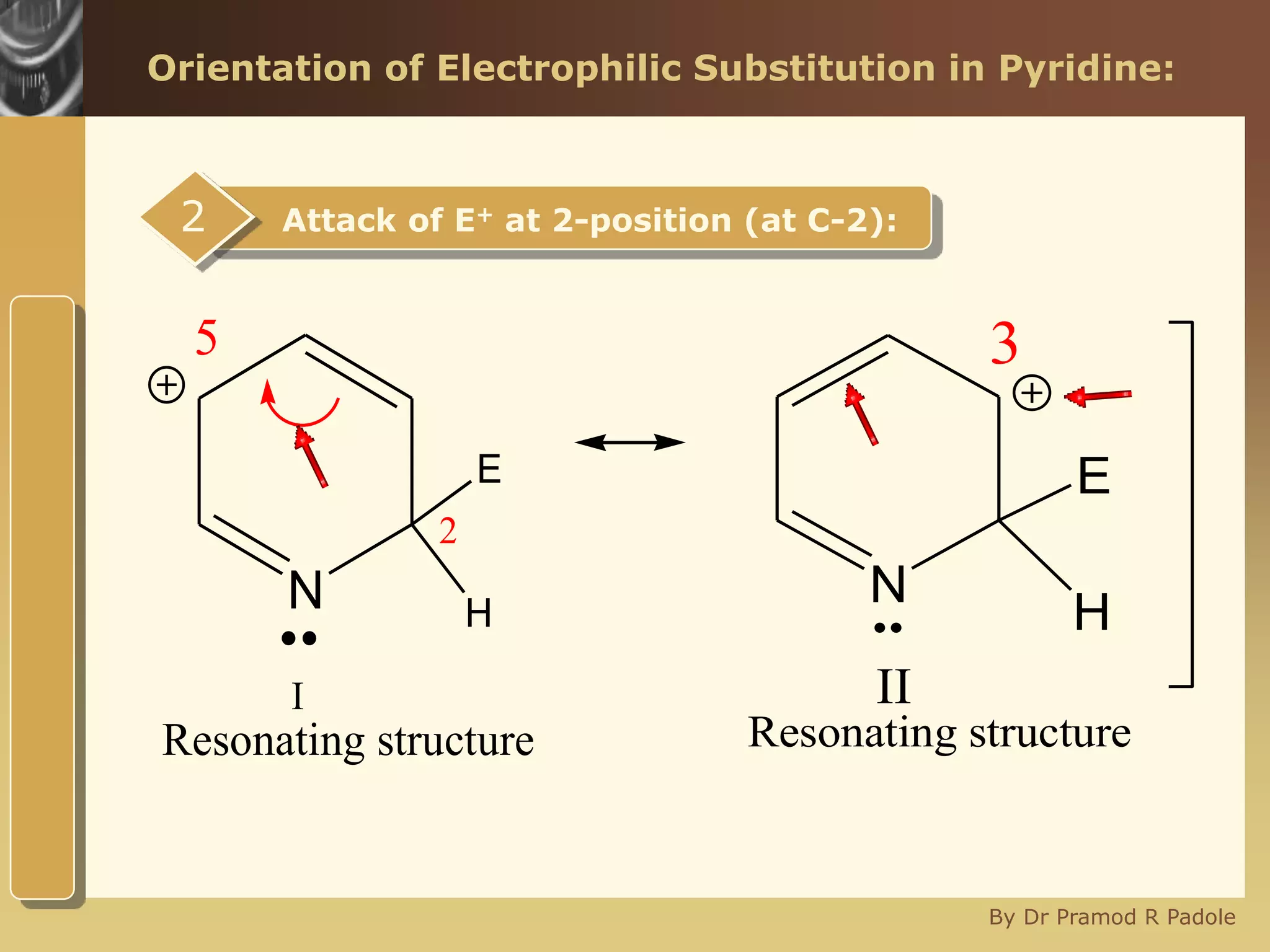
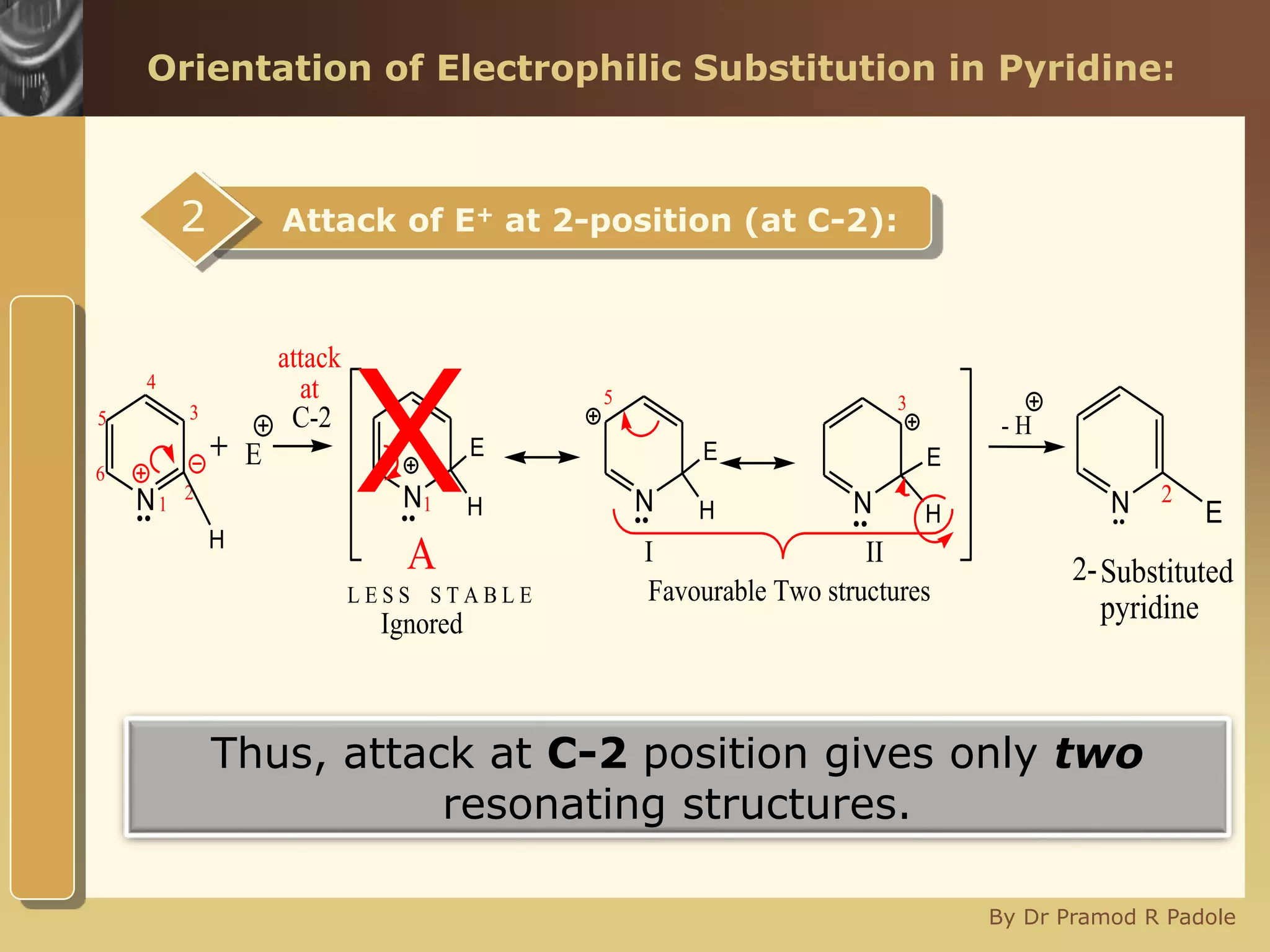
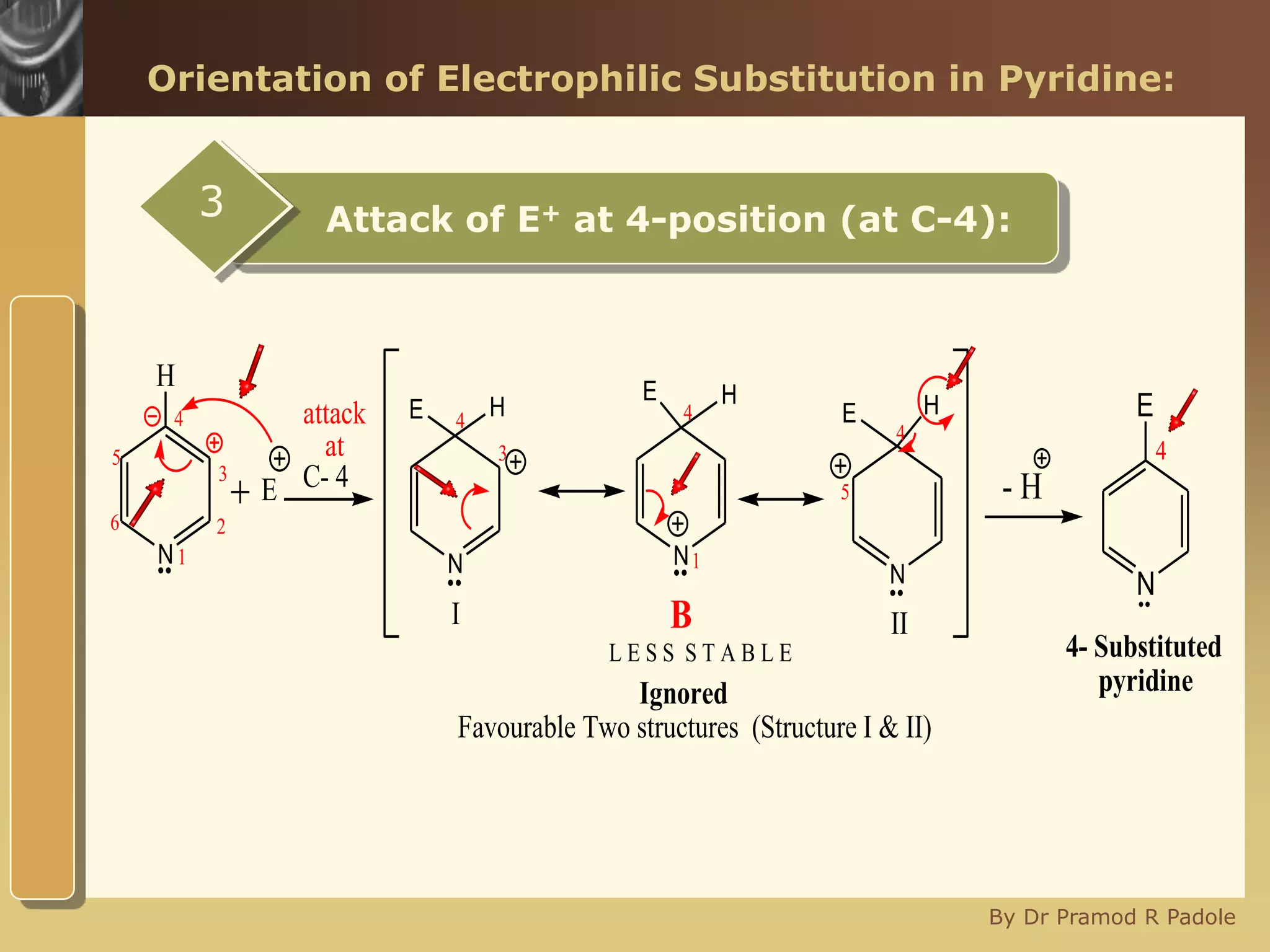
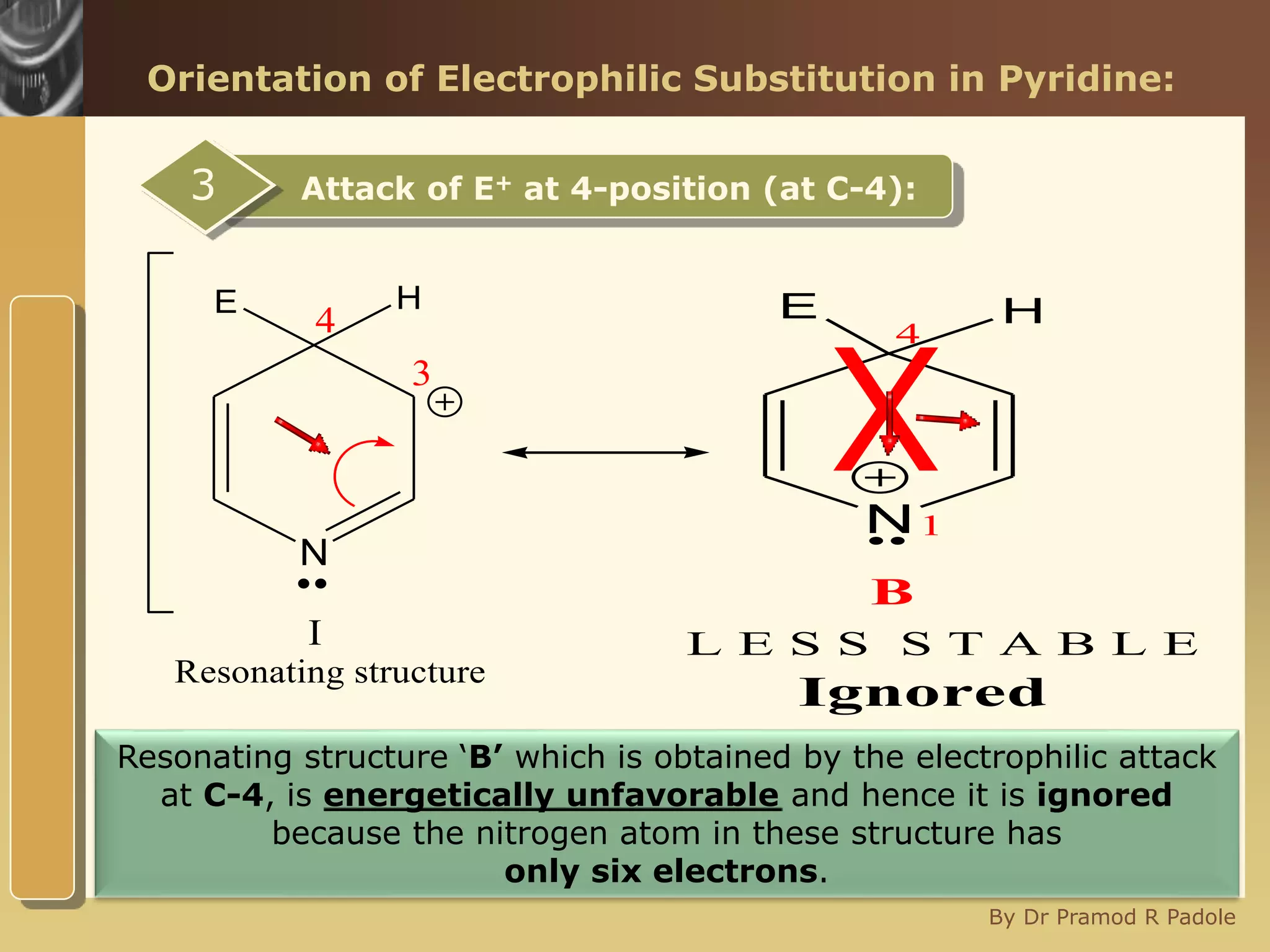
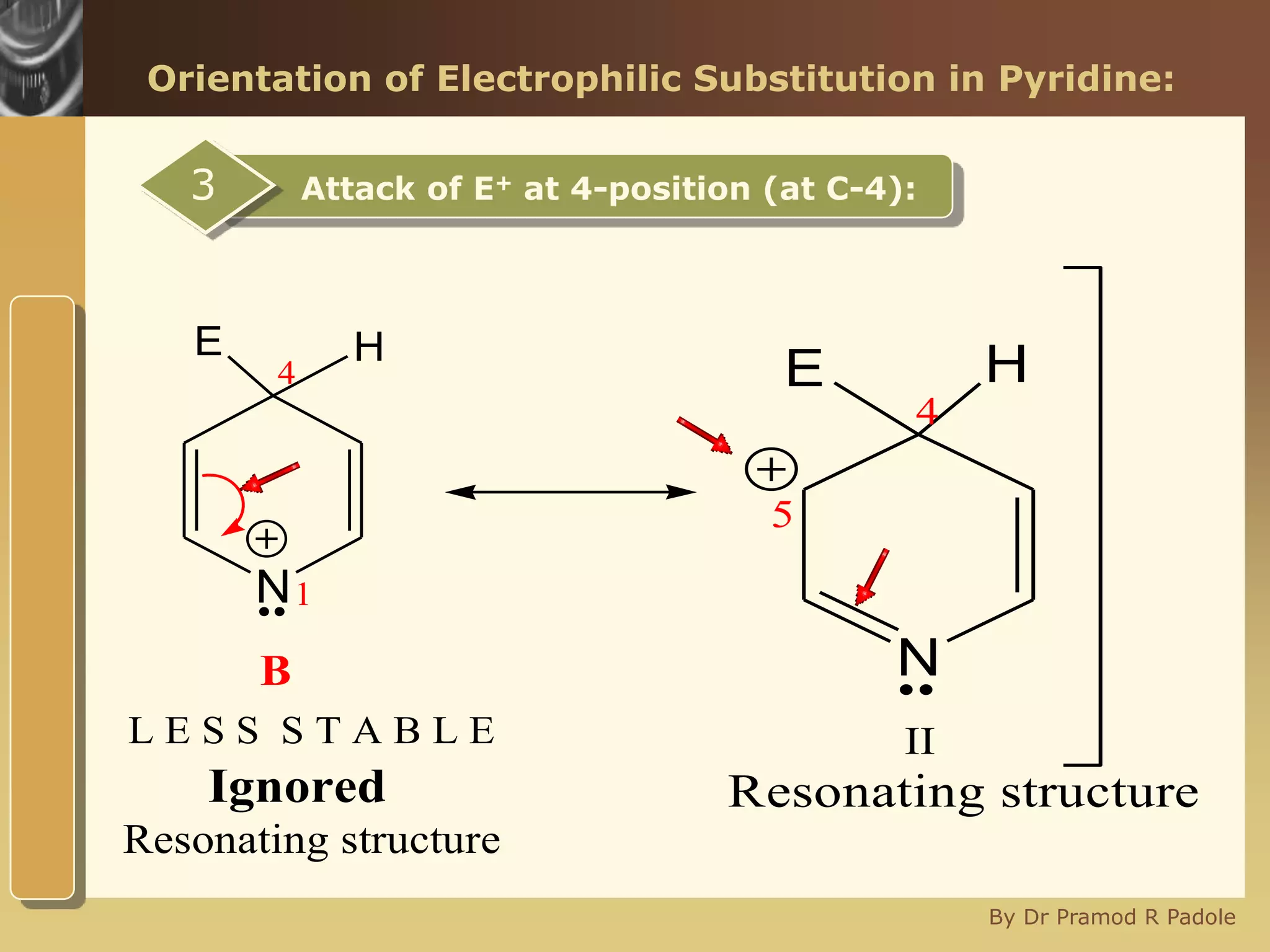
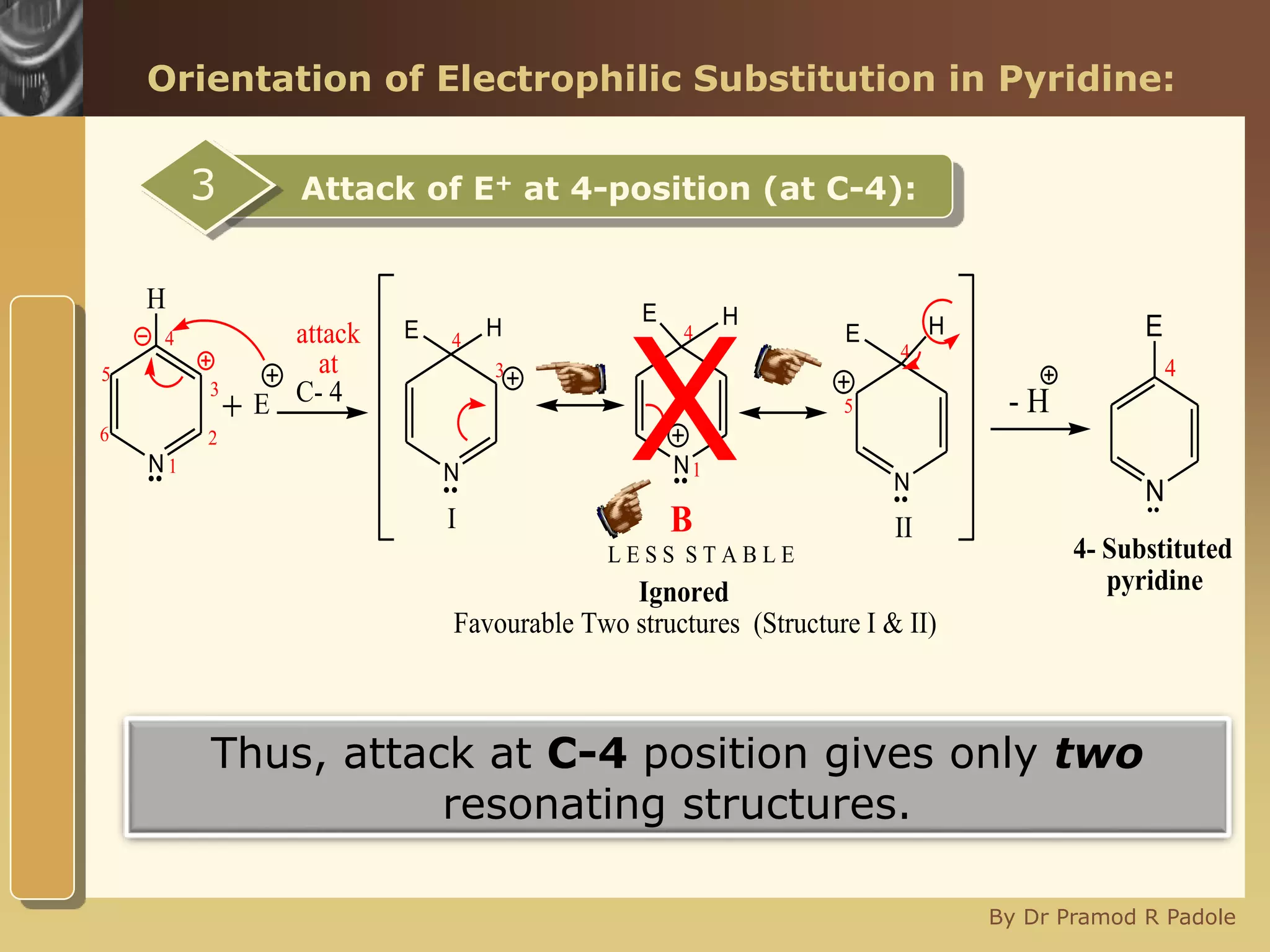
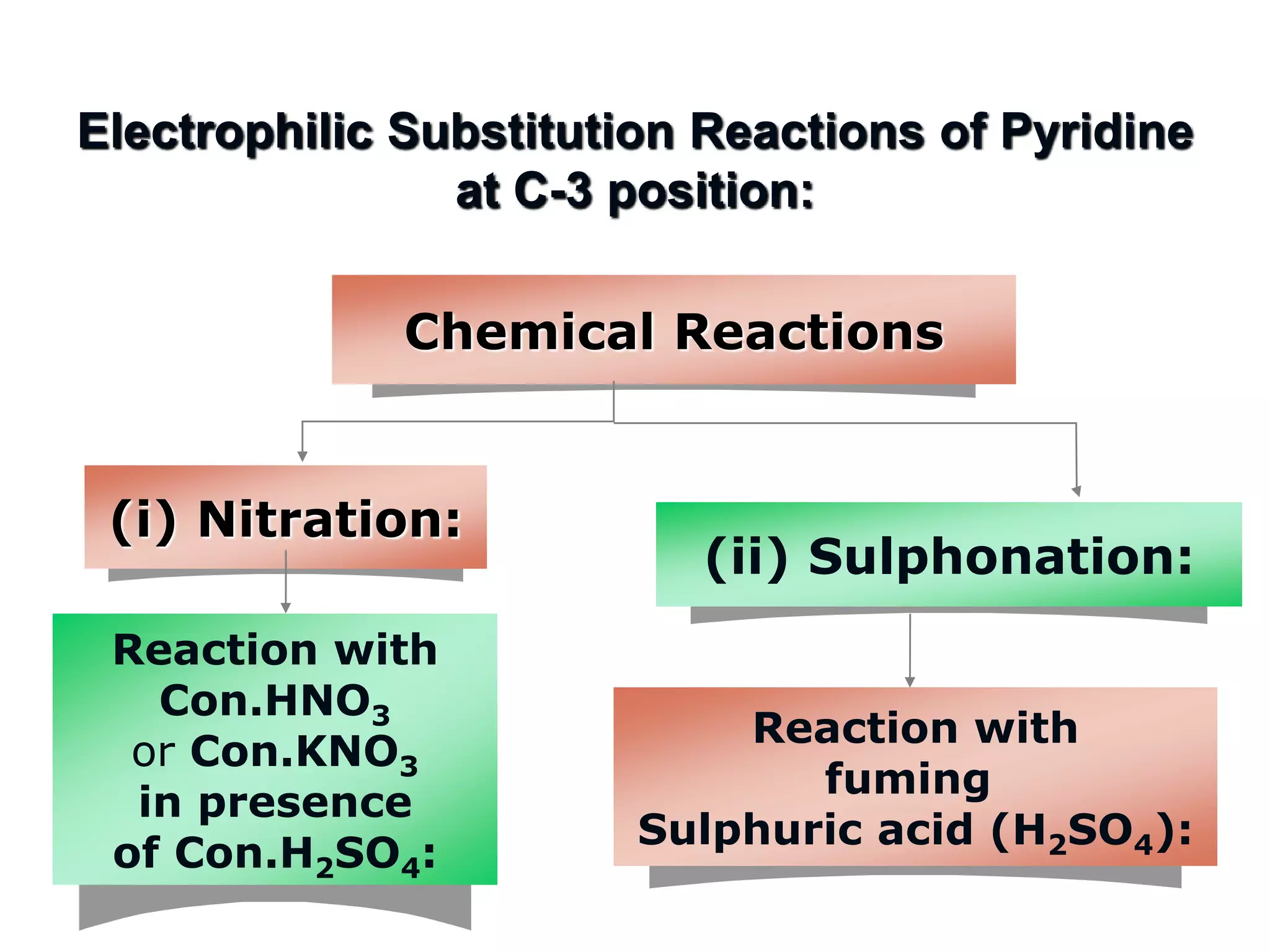
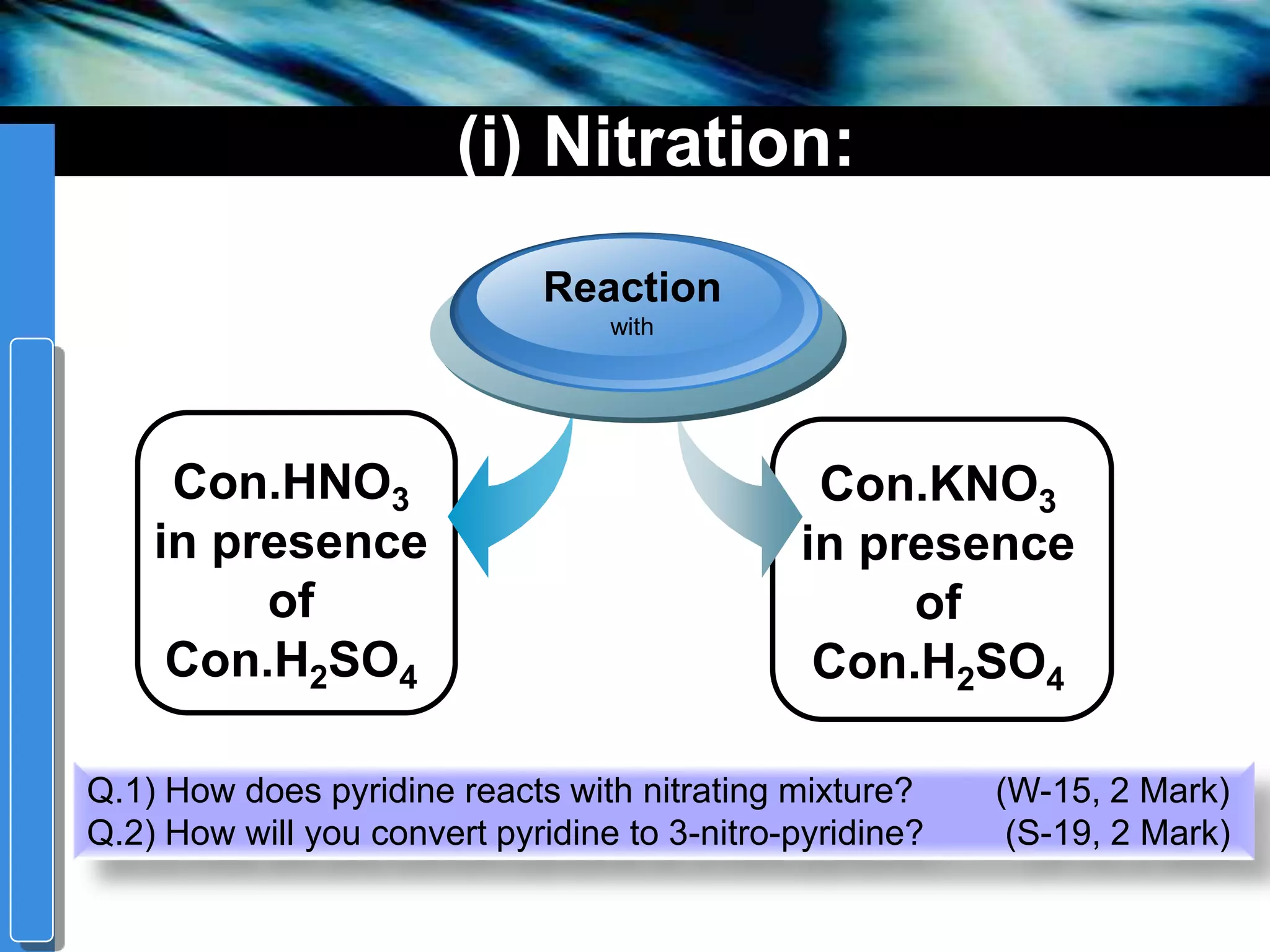
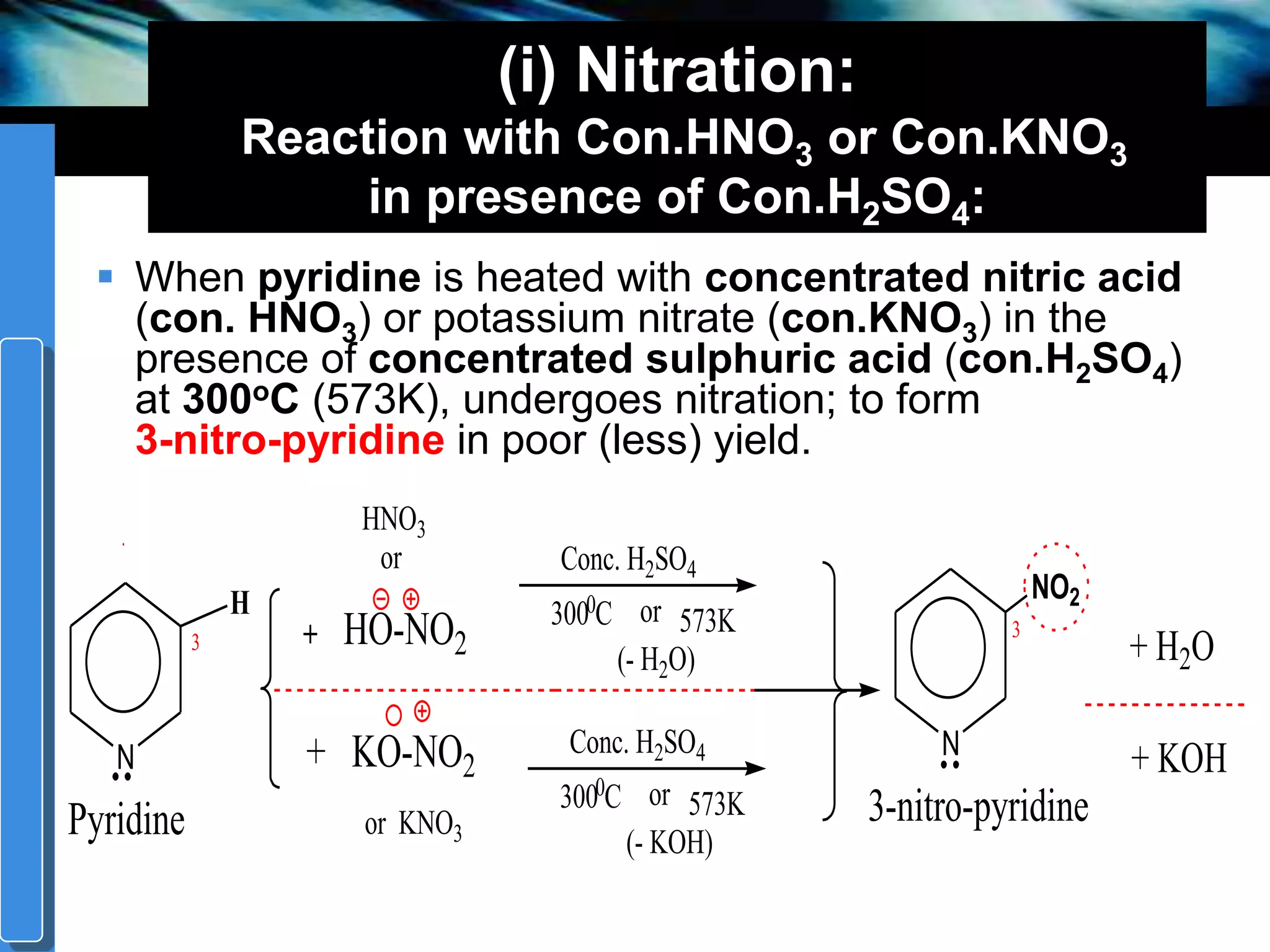
The document discusses the electrophilic substitution reactions in pyridine, emphasizing that such reactions predominantly occur at the 3-position due to the stability of the intermediate carbonium ion formed at that position. It highlights that pyridine is less reactive towards electrophilic substitution compared to benzene due to the electronegativity of nitrogen, which decreases the electron density in the ring. The document also explains why reactions at the 2 and 4 positions are less favorable, outlining the mechanisms and conditions for nitration and sulfonation of pyridine.