Embed presentation
Downloaded 19 times

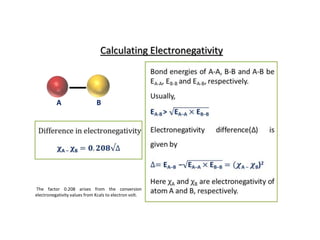

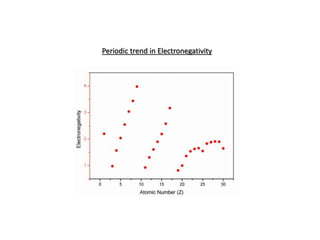

Linus Pauling defined electronegativity as "the power of an atom in a molecule to attract electrons to itself." He developed a method to calculate electronegativity values based on the difference between the measured and theoretical bond energies. The theoretical bond energy is calculated as the square root of the product of the atomic electron affinities. Electronegativity generally increases along periods in the periodic table and decreases down groups, with fluorine being the most electronegative element. Bonds between atoms with similar electronegativity values (<1.7 difference) are predominantly covalent or metallic, while bonds between atoms with larger differences (>1.7) are predominantly ionic.





