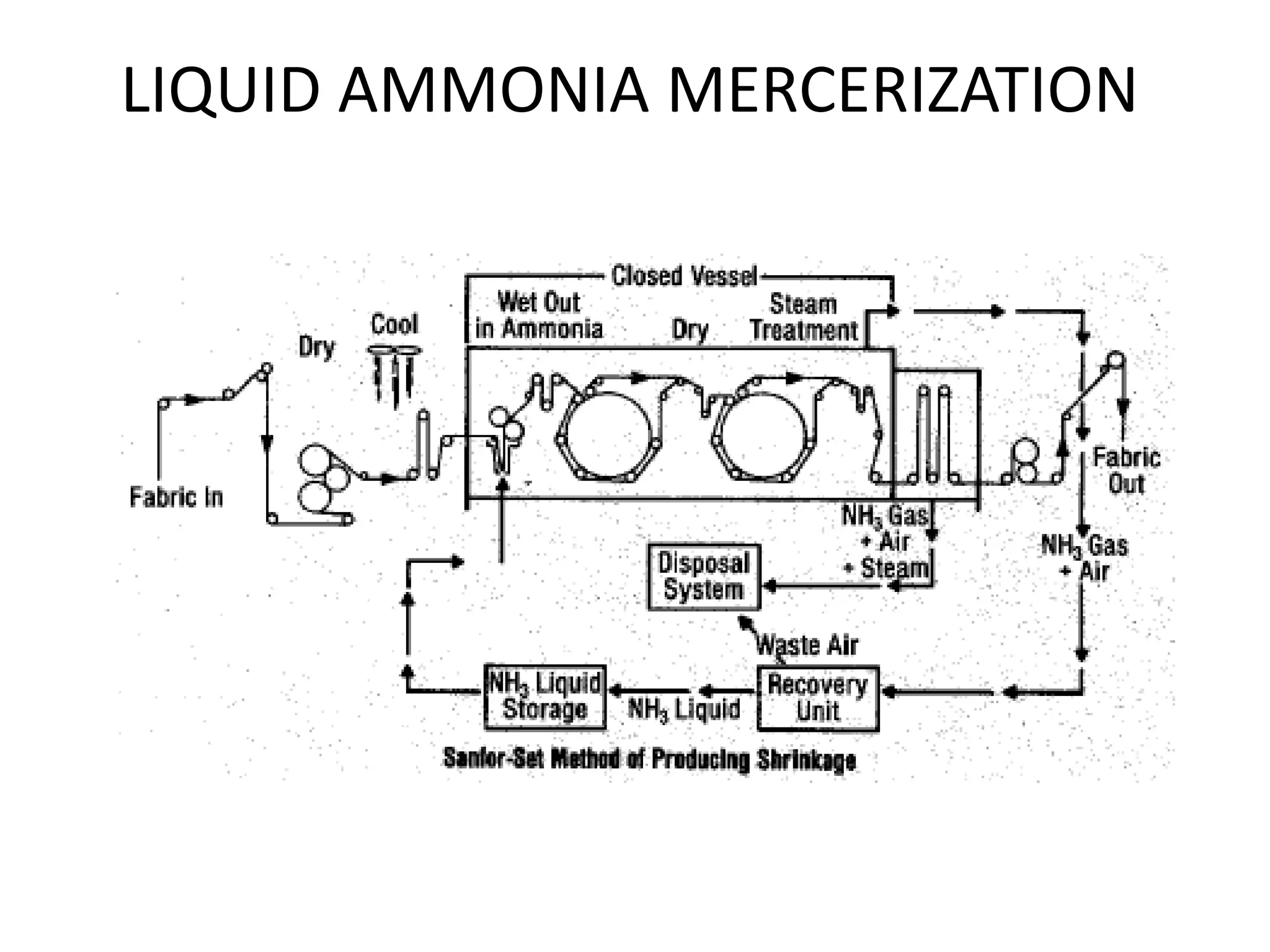
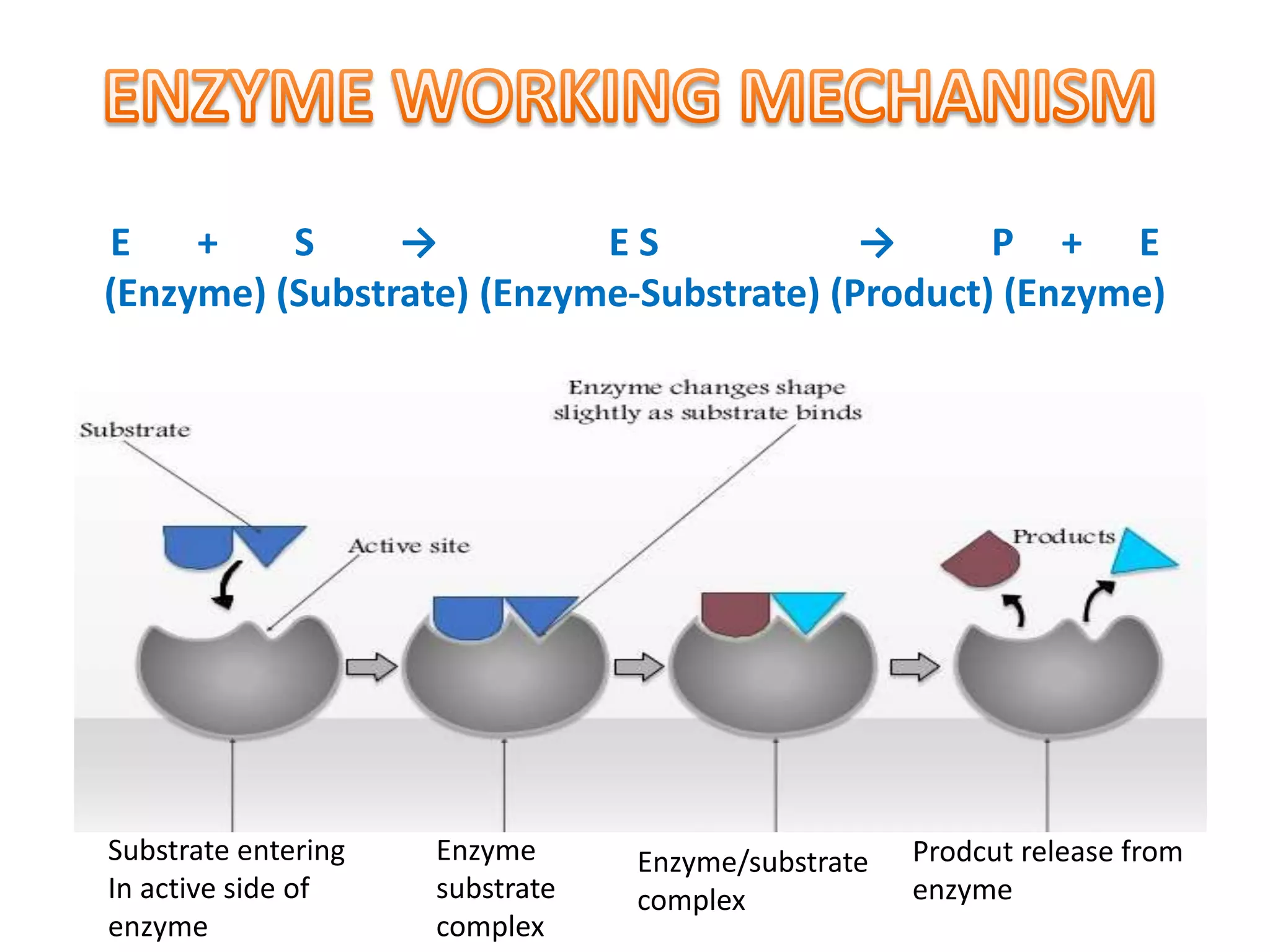
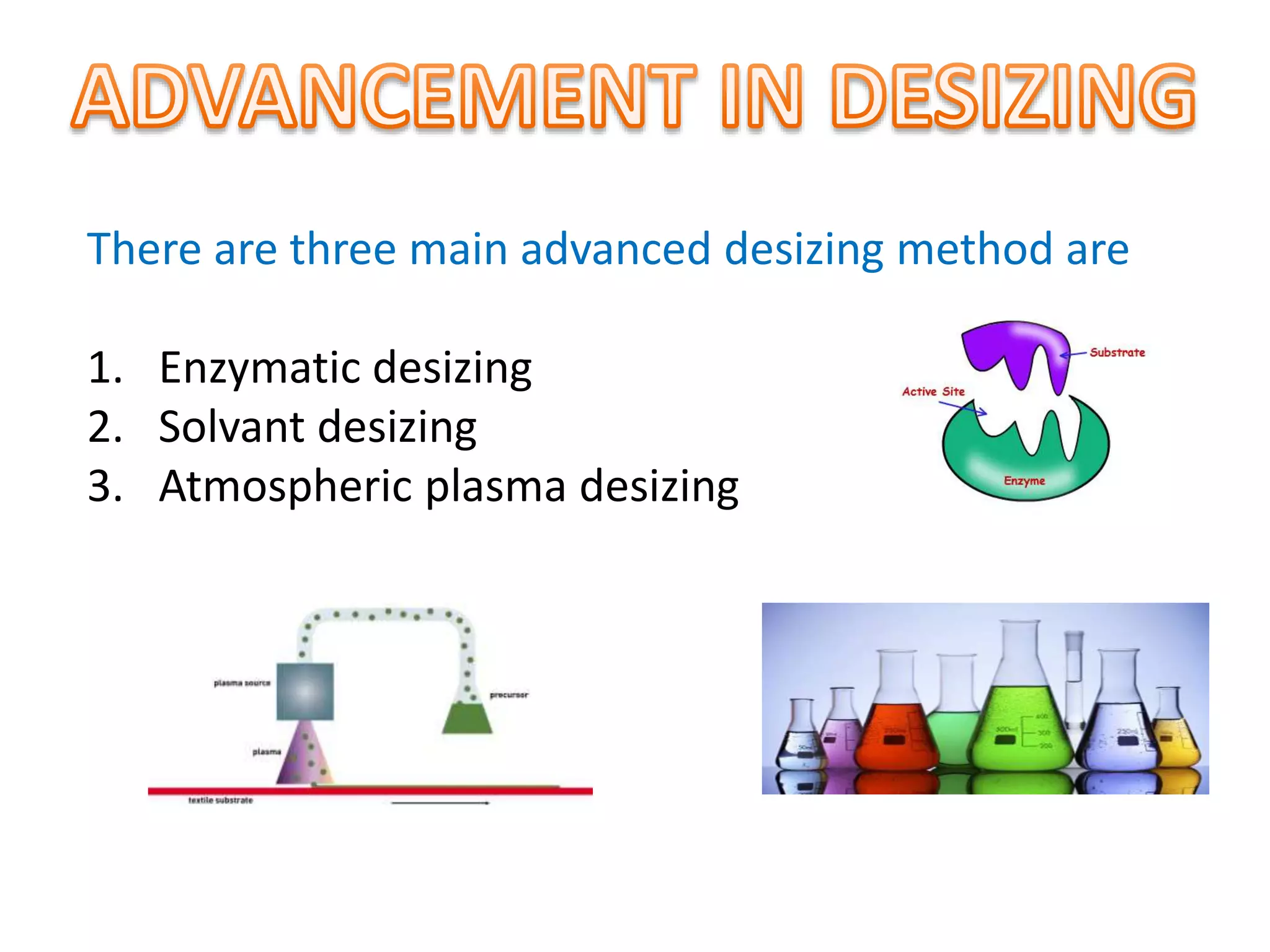
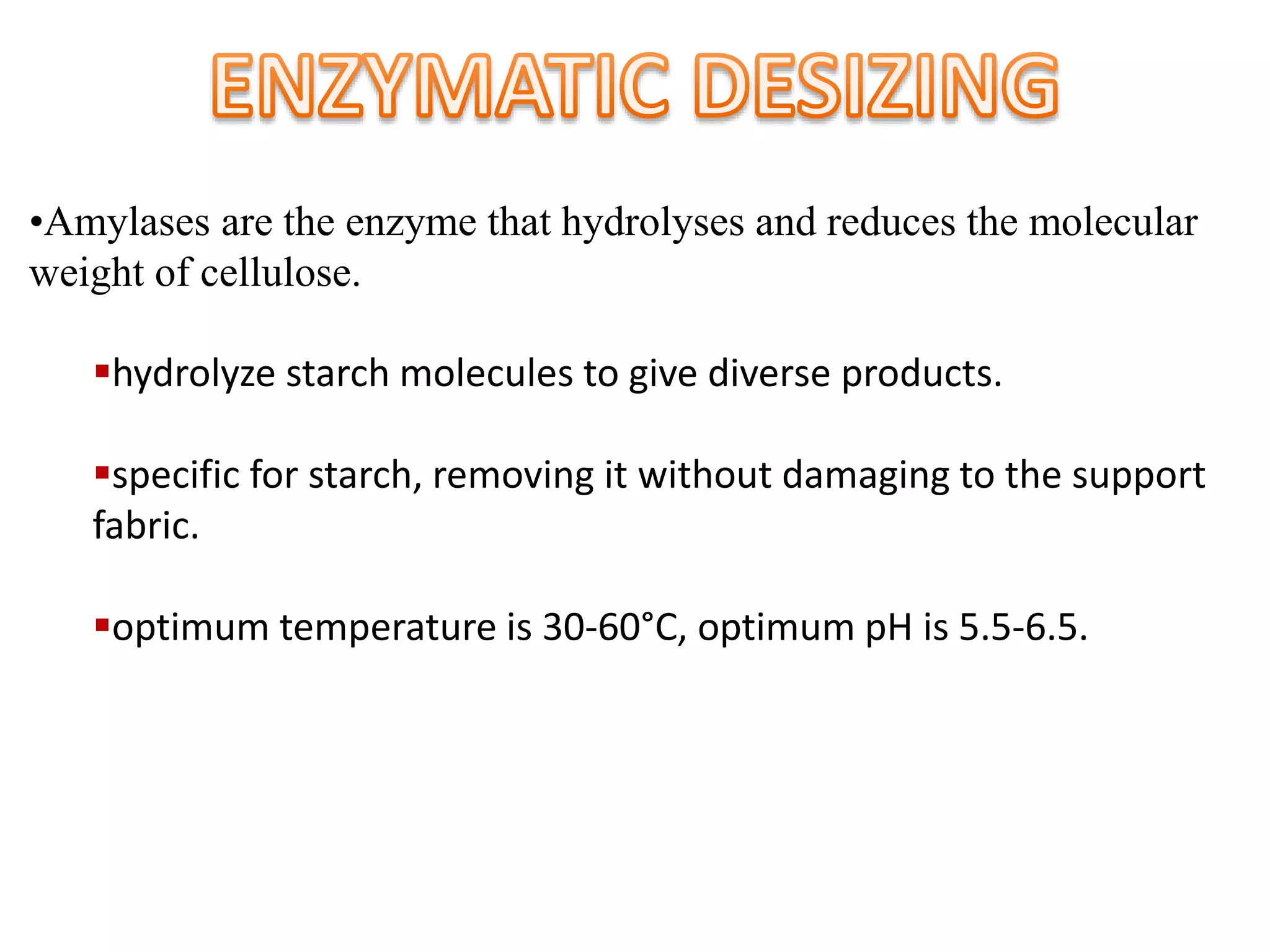
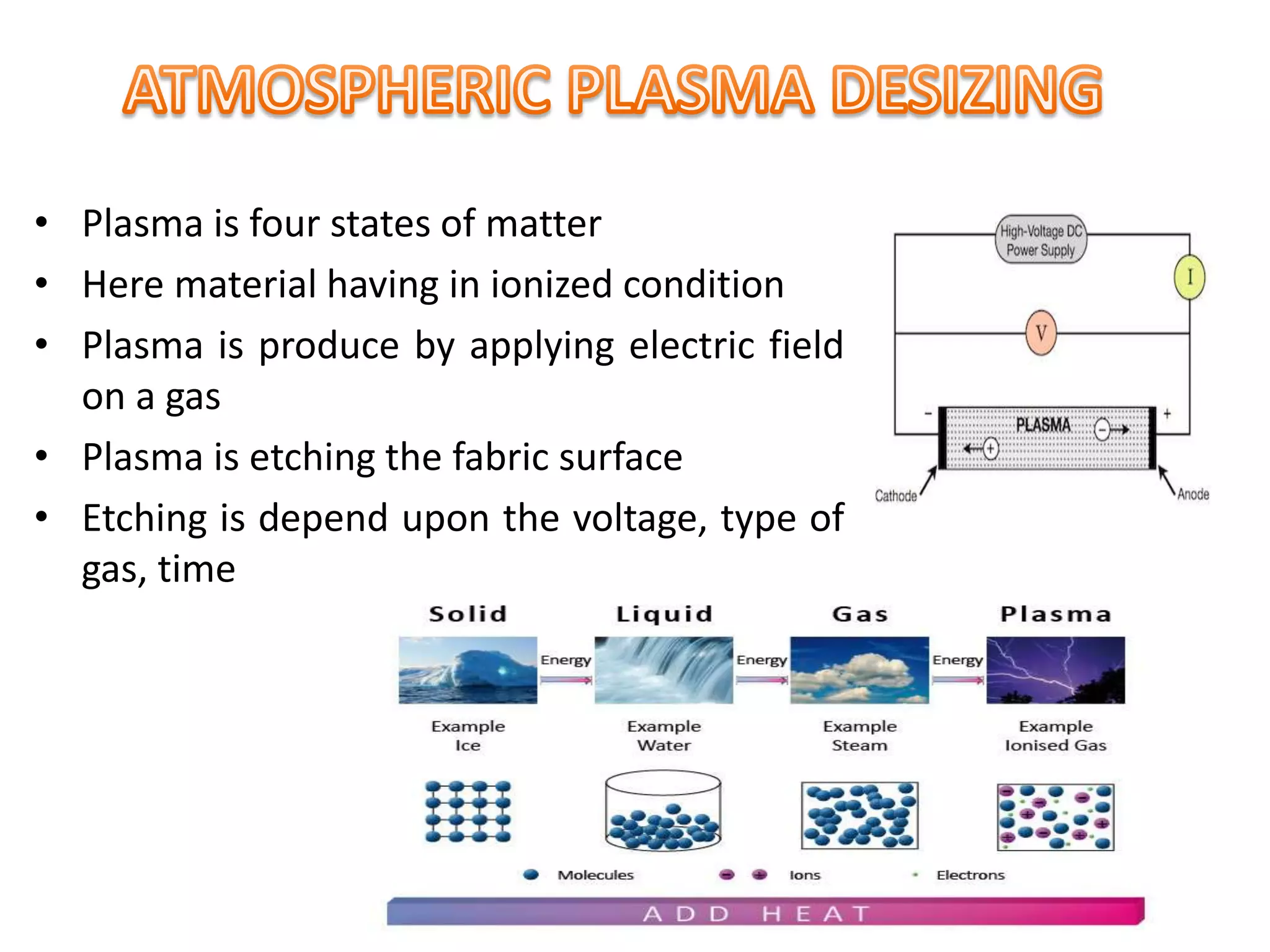
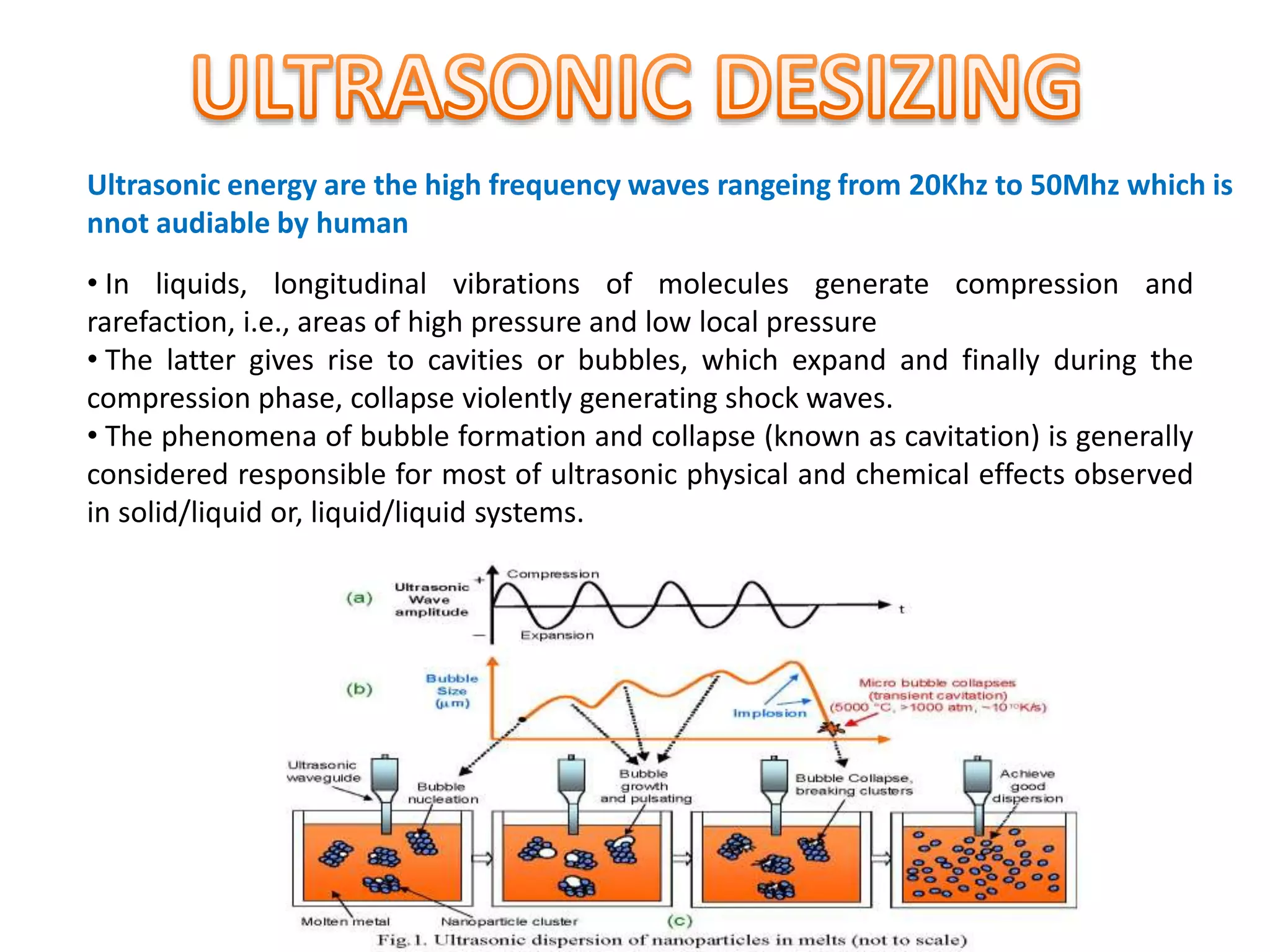
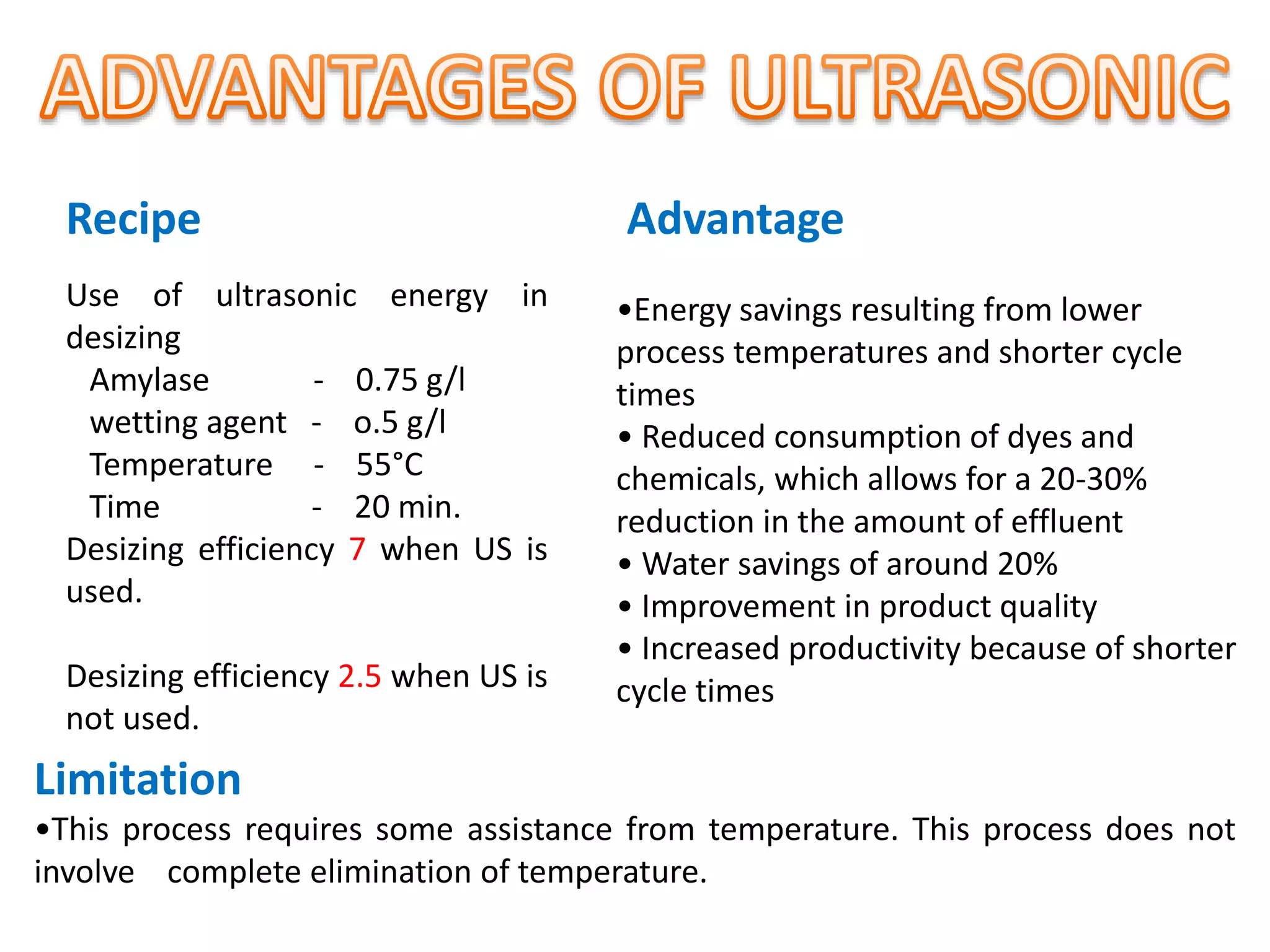
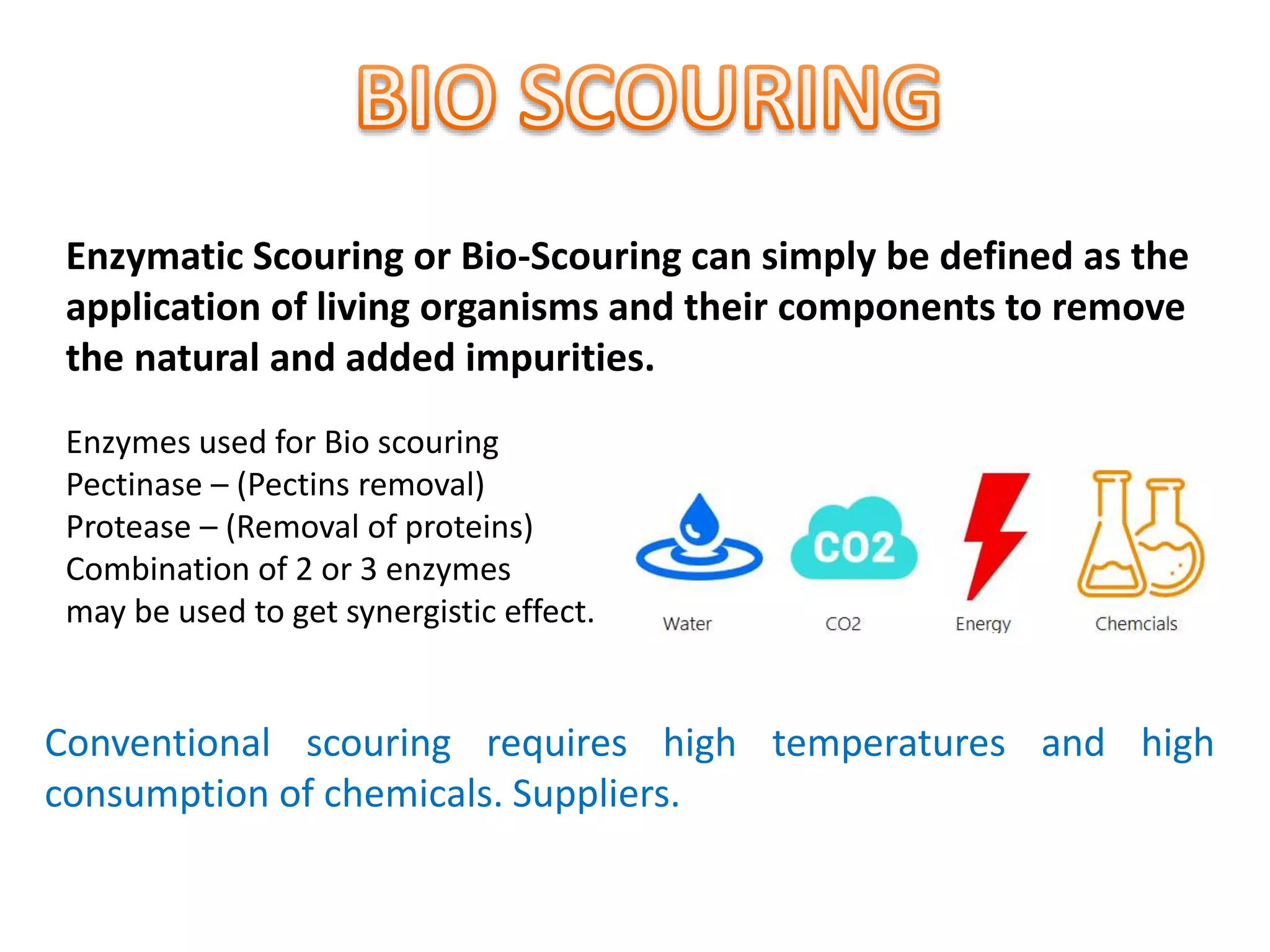
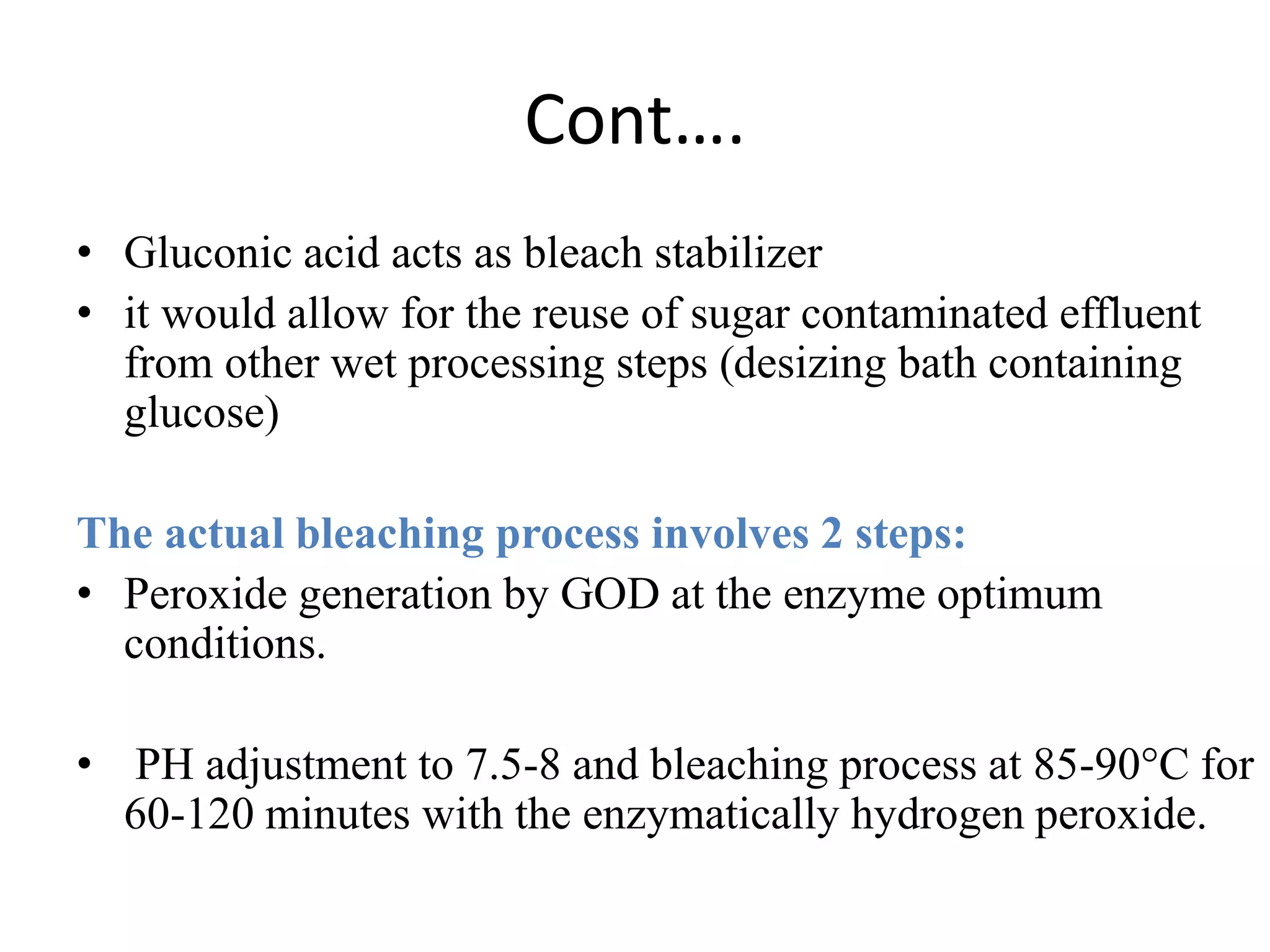
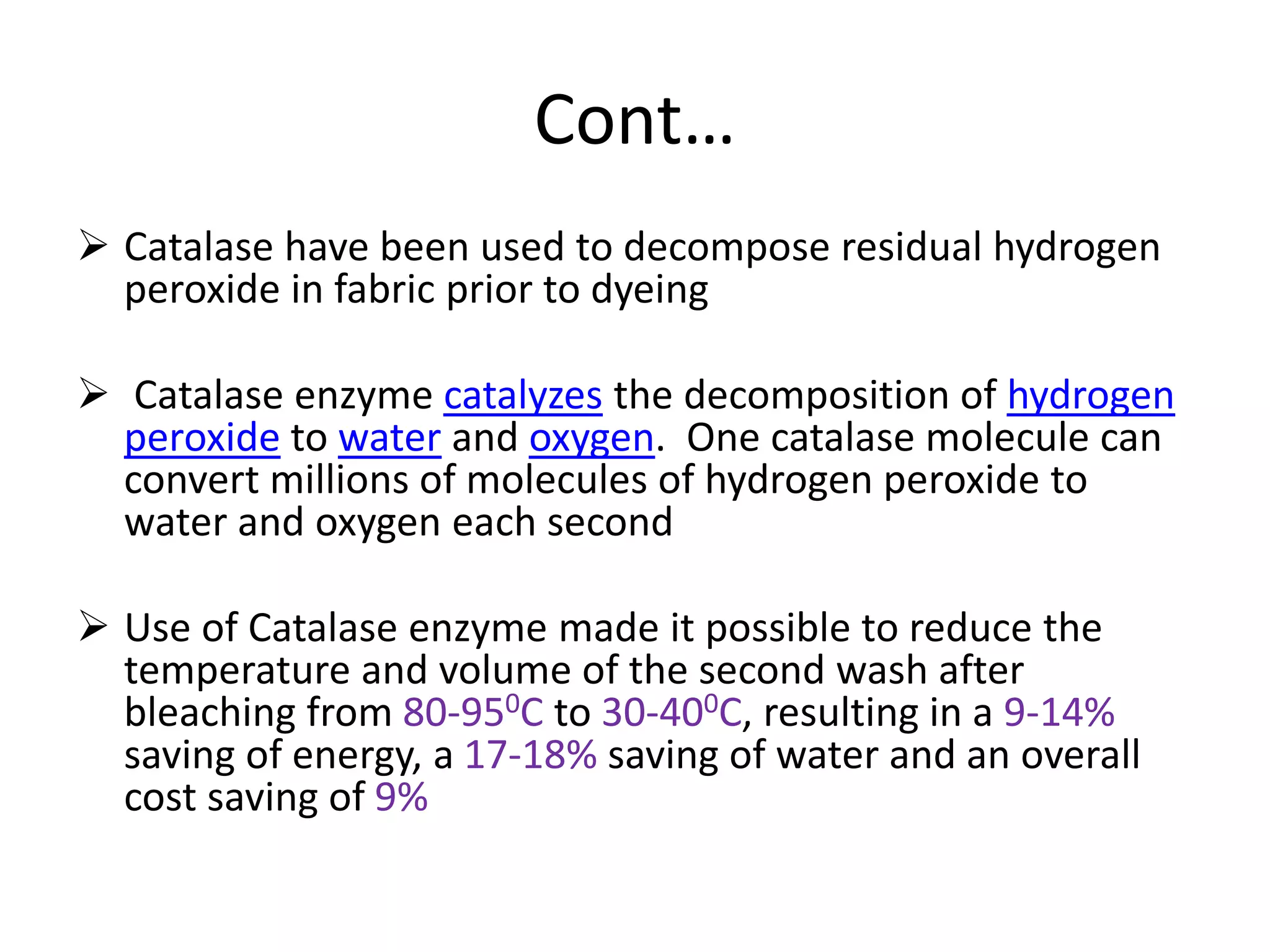
The document discusses eco-friendly textile processing methods under the guidance of Prof. M.D. Teli, emphasizing the removal of impurities and enhancing fabric quality through various techniques such as enzymatic desizing, bio-scouring, and ozone bleaching. It outlines the advantages of using enzymes and alternative materials for reducing environmental impacts while detailing procedures for effective processing. The conclusion highlights the urgent need for the textile industry to adopt sustainable practices amid increasing environmental regulations.























![Mechanism of oxidation with peracetic acid, it gives extra
oxygen atom and acetic acid is formed as co-product.
CH3COOH CH3COOH + [O]
Procedure for bleaching with peracetic acid:
1) Peracetic acid being unstable has to be prepared before bleaching being
carried out as per above procedure.
2) Bleaching is carried out with
MECHANISM OF PERACETIC ACID](https://image.slidesharecdn.com/advecofreindlypretrtrt-160421074340/75/eco-friendly-textile-processing-34-2048.jpg)