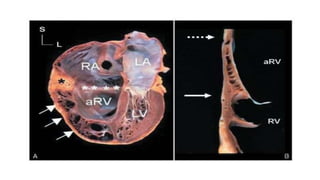
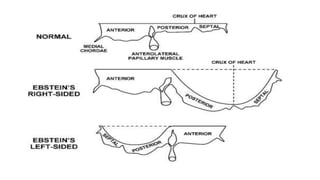
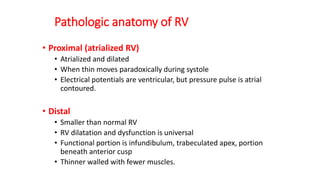
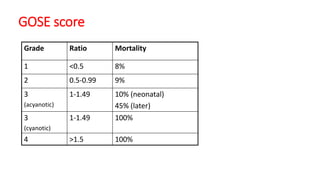
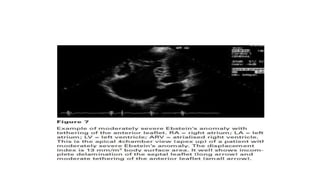
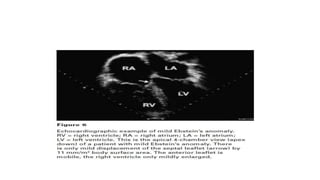
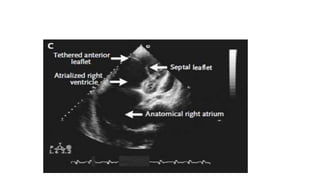
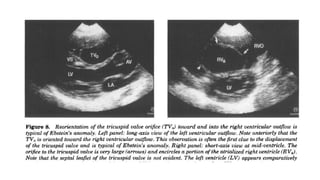
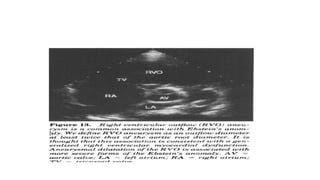
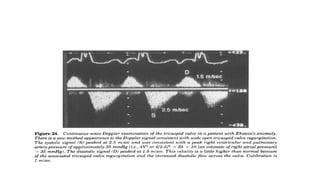
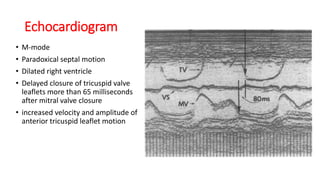
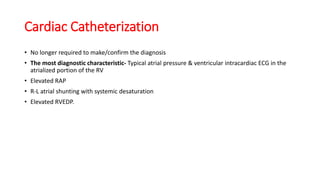
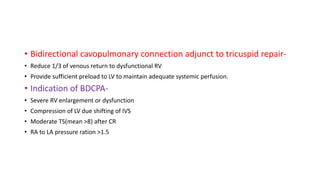
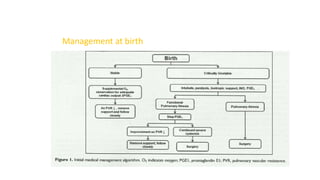
Ebstein anomaly is a rare congenital heart defect characterized by abnormal development of the tricuspid valve and the right ventricle, with a prevalence of 0.3-0.8% among congenital heart diseases. The condition has significant associated risks, particularly in neonates, with survival rates varying based on severity and associated anomalies, while treatments may involve medical management or surgical interventions, including valve repair or replacement. Regular monitoring and specialized care are essential for the management of patients with Ebstein anomaly, particularly for those experiencing arrhythmias and heart failure.