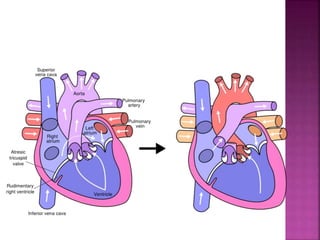
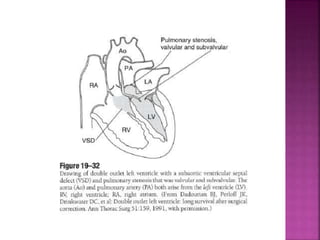
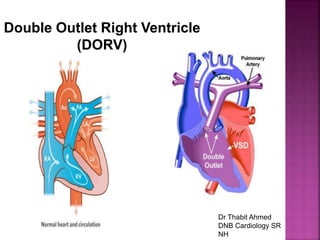
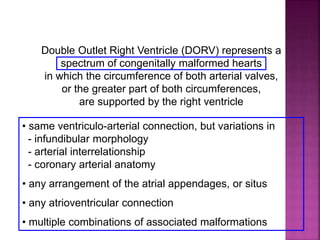
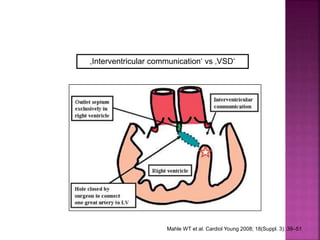
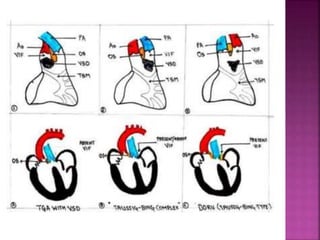
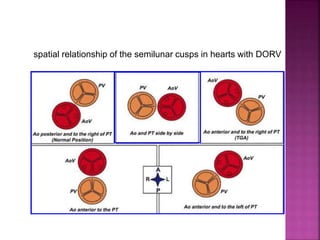
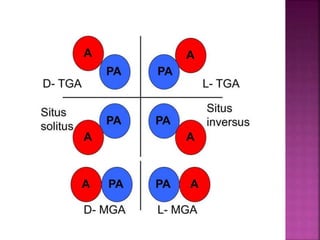
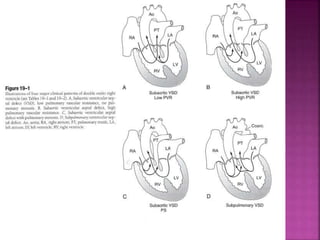
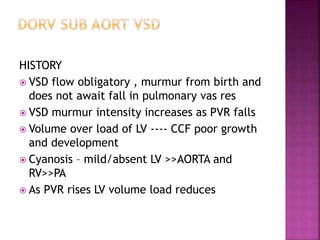
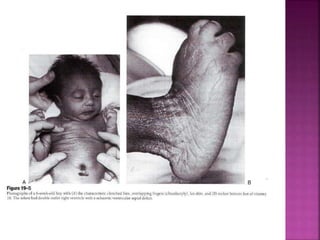
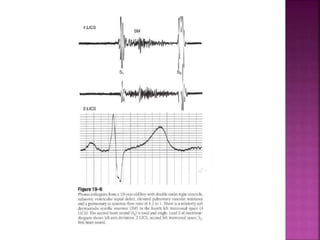
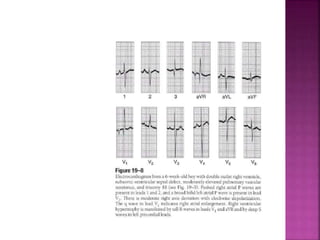
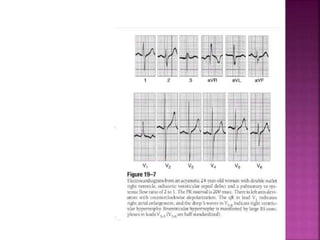
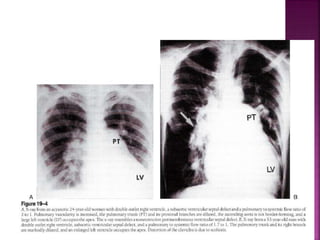
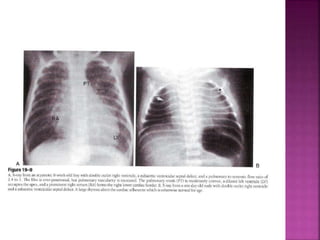
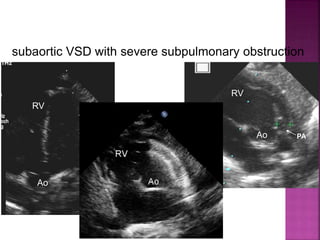
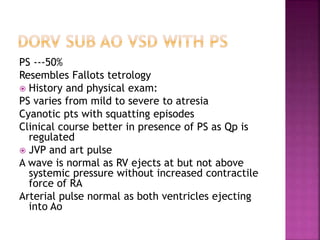
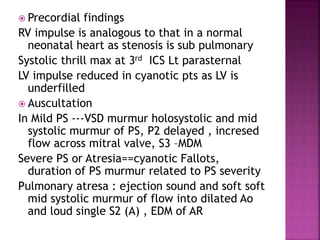
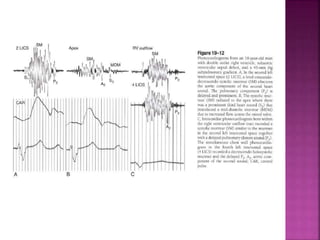
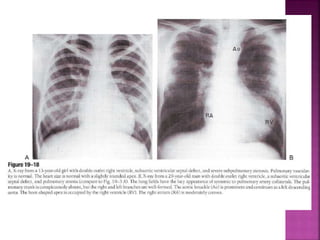
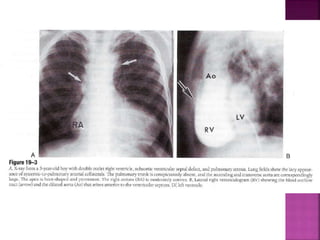
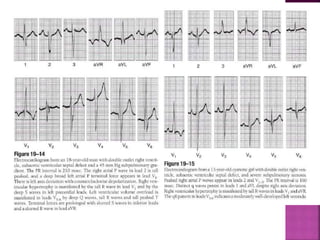
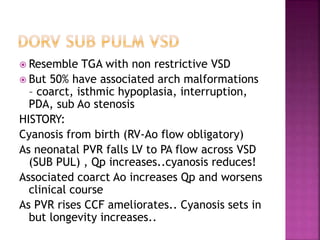
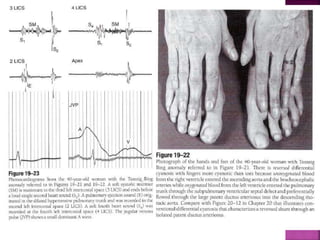
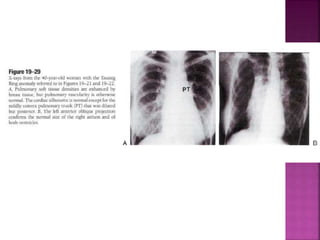
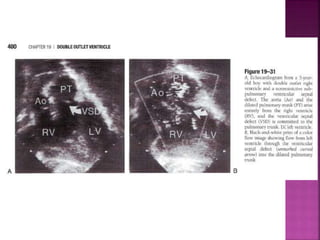
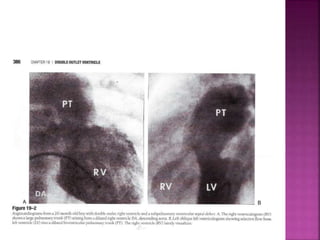
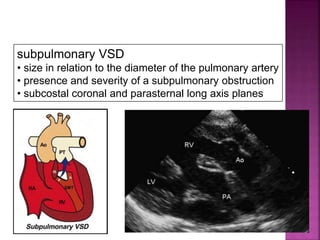
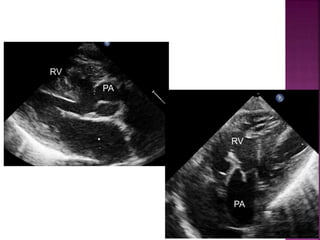
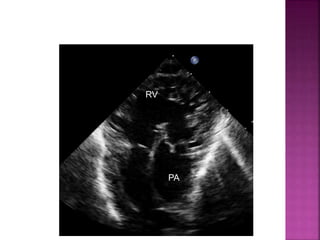
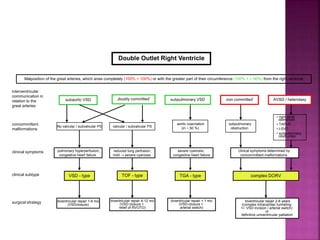
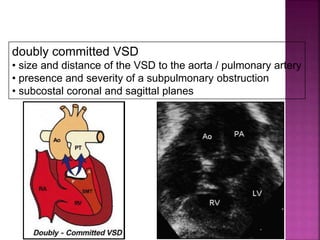
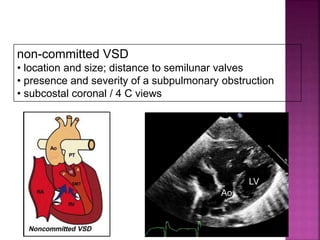
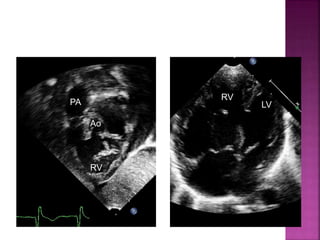
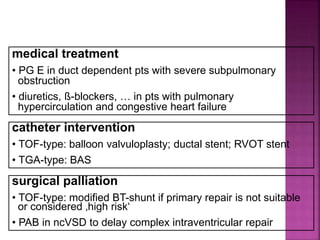
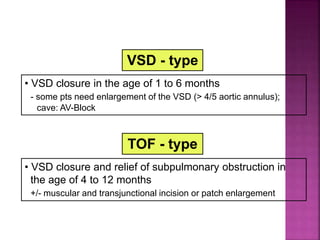
Double outlet right ventricle (DORV) is a heart defect where both the aorta and pulmonary artery arise completely or primarily from the right ventricle. It can cause varying degrees of cyanosis and congestive heart failure depending on pulmonary pressures and associated defects. Echocardiography is important for assessing the relationship of the great vessels to the ventricles, presence of a ventricular septal defect, and other structural issues to determine appropriate surgical repair. Management may involve biventricular repair in the neonatal period or staged palliation depending on the specific anatomy and physiology in each case.
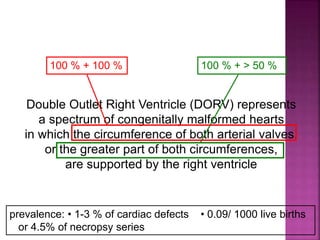
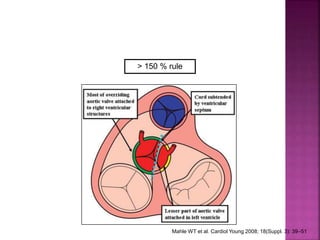
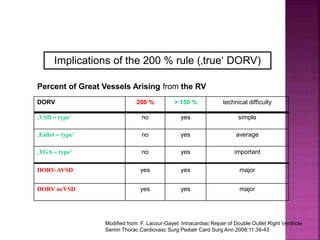
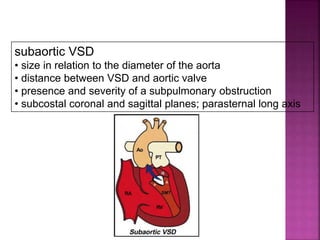
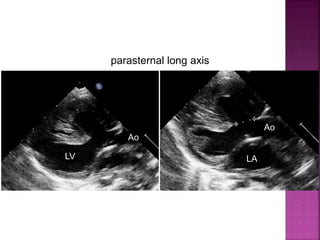
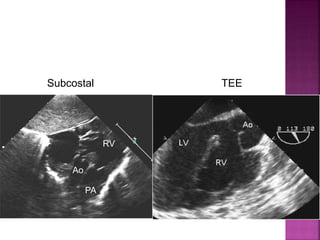
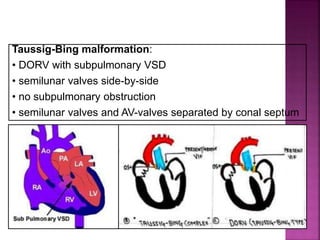
![ 50% Rule(At least 50% each GA’s from RV)
200% rule(Both the GA’s completely from RV)
Aortomitral discontinuity is must
[ To differentiate from TOF with significant aortic
override but maintained aortomitral continuity ]](https://image.slidesharecdn.com/dorvthab-160406061609/85/Dorv-thab-4-320.jpg)




















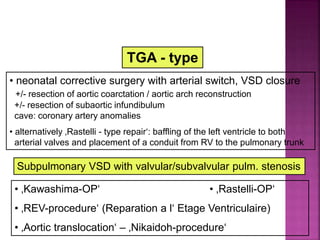

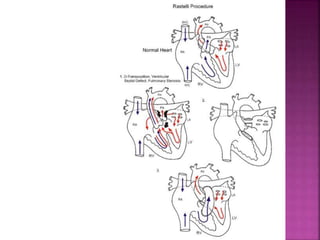
![ Rastelli repair
REV procedure
The REV procedure (réparation à l’étage
ventriculaire) entails division of the main PA with
extensive mobilization,translocation of the PA
anterior to aorta (Lecompte maneuver),and
direct connection of the PA to the RV, thus
eliminating the use of prosthetic materials]
Nikaidoh repair
The Nikaidoh procedure is an aortic root
translocation procedure into the enlarged
pulmonary root position. A right ventricle-to-
pulmonary artery (RV-PA) conduit is then placed].](https://image.slidesharecdn.com/dorvthab-160406061609/85/Dorv-thab-70-320.jpg)