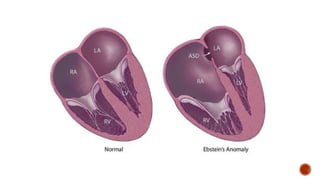
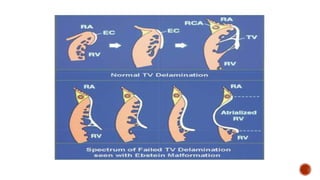
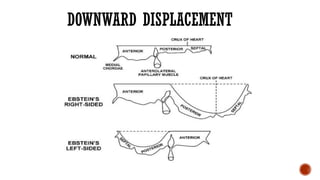
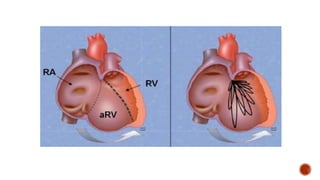
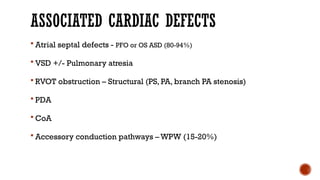
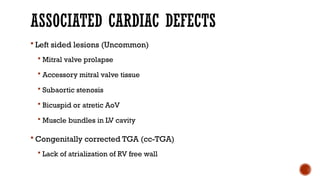
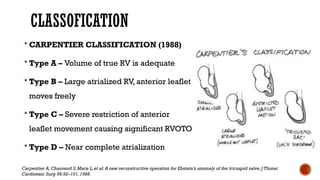
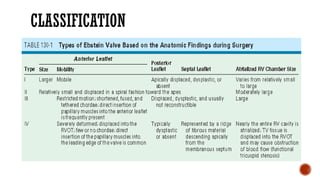
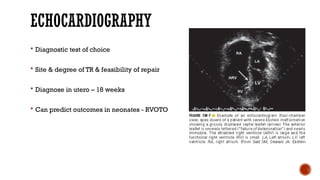
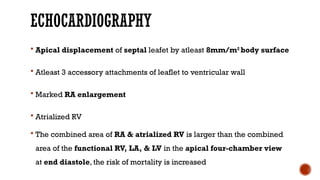
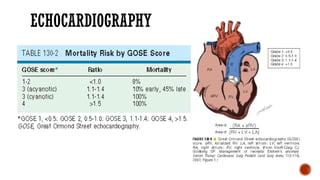
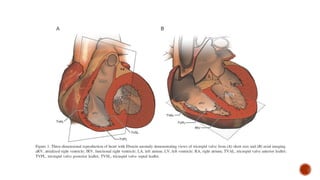
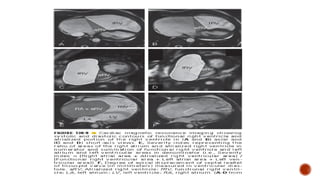
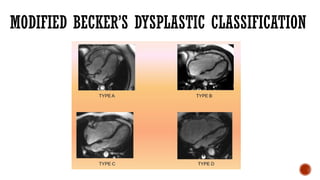
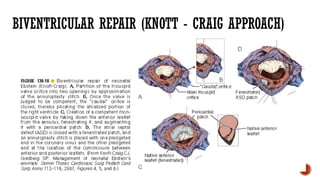
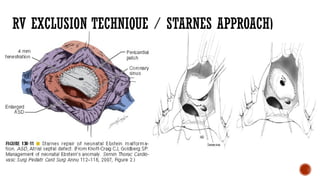
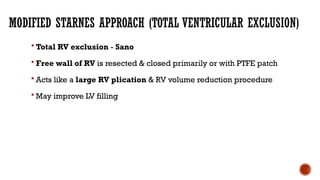
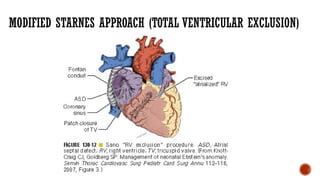
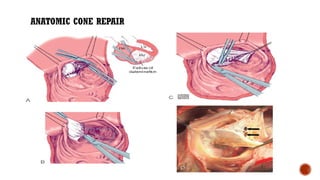
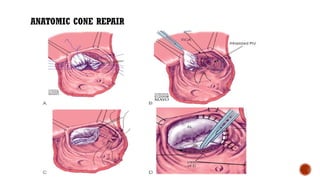
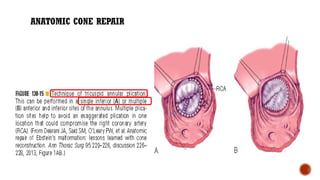
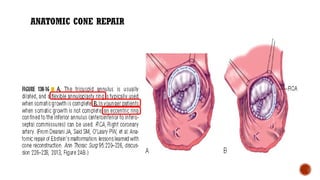
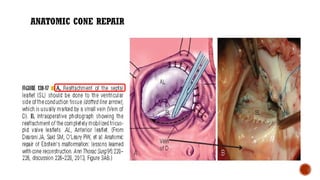
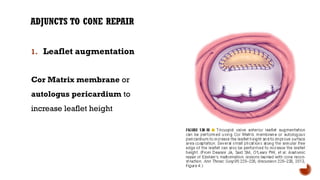
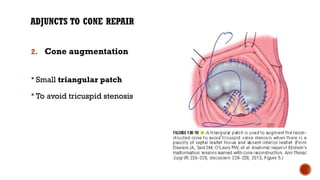
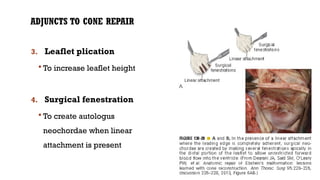
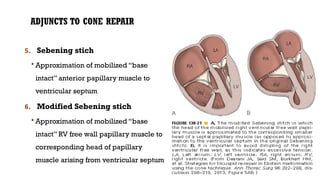
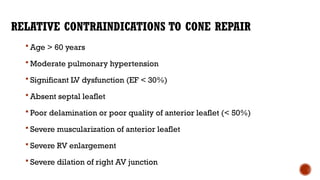
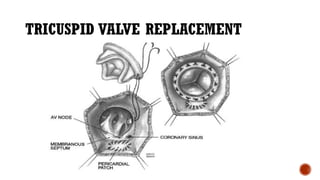
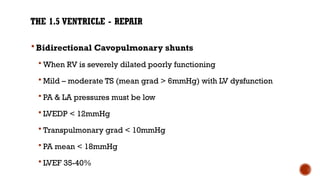
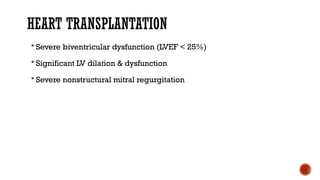
Ebstein's anomaly, first described in 1866, is characterized by malformation of the tricuspid valve leading to atrial enlargement and right ventricular dysfunction. Clinical presentations vary by age, including cyanosis and heart failure in infants and arrhythmias in older patients, while imaging techniques such as echocardiography and cardiac MRI are critical for diagnosis. Management options range from surgery to heart transplantation, depending on severity and symptomatology.