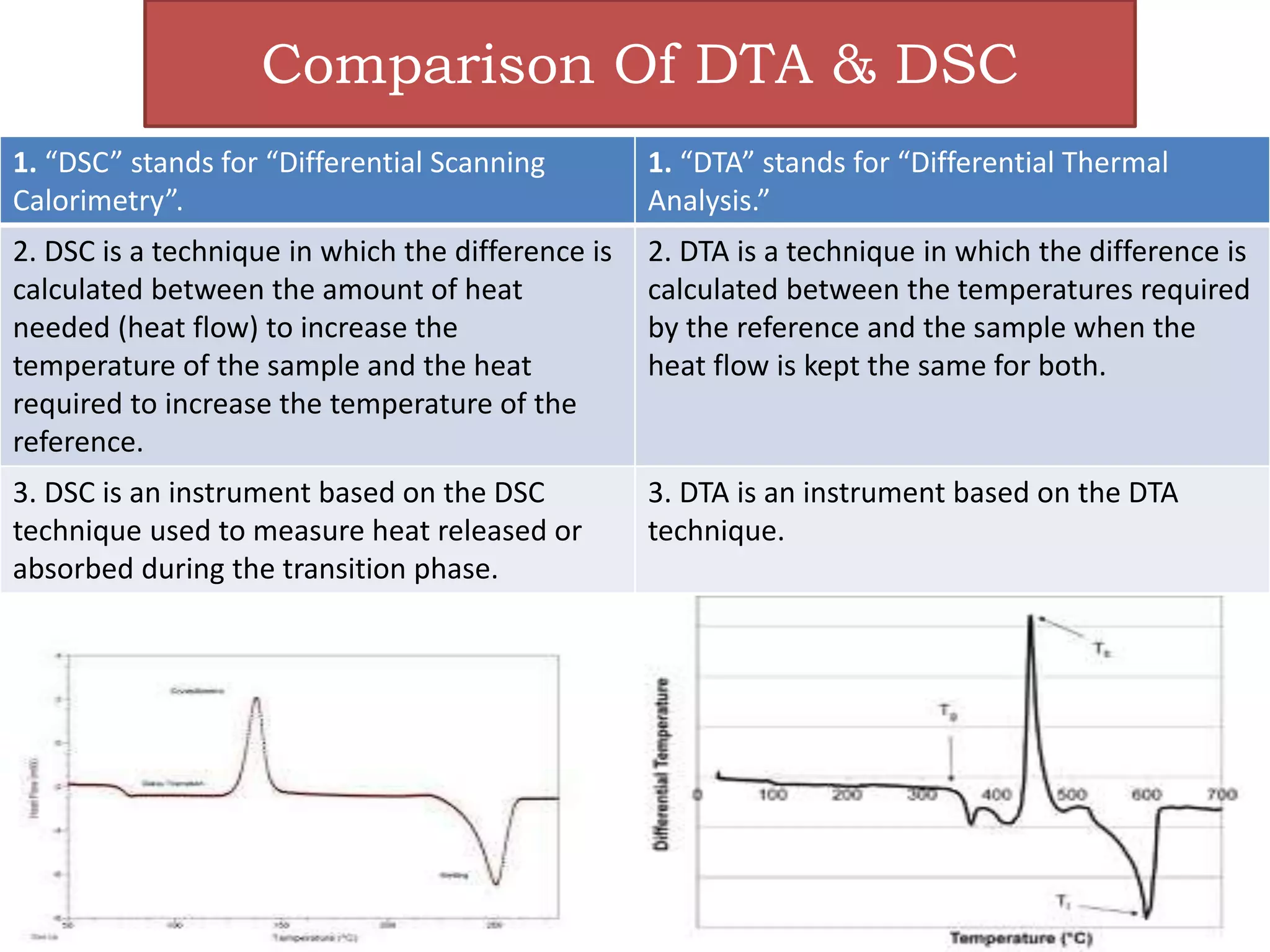
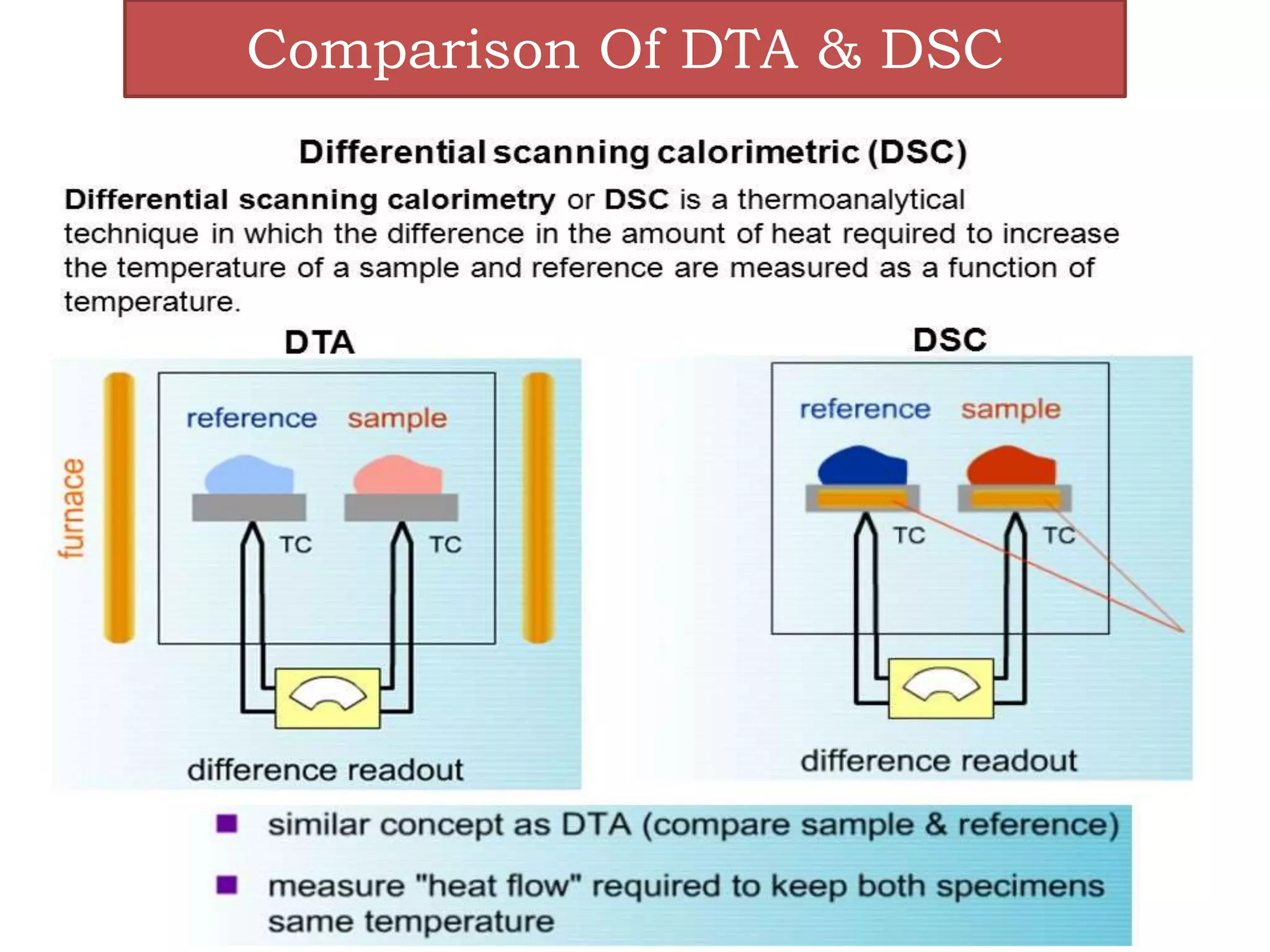
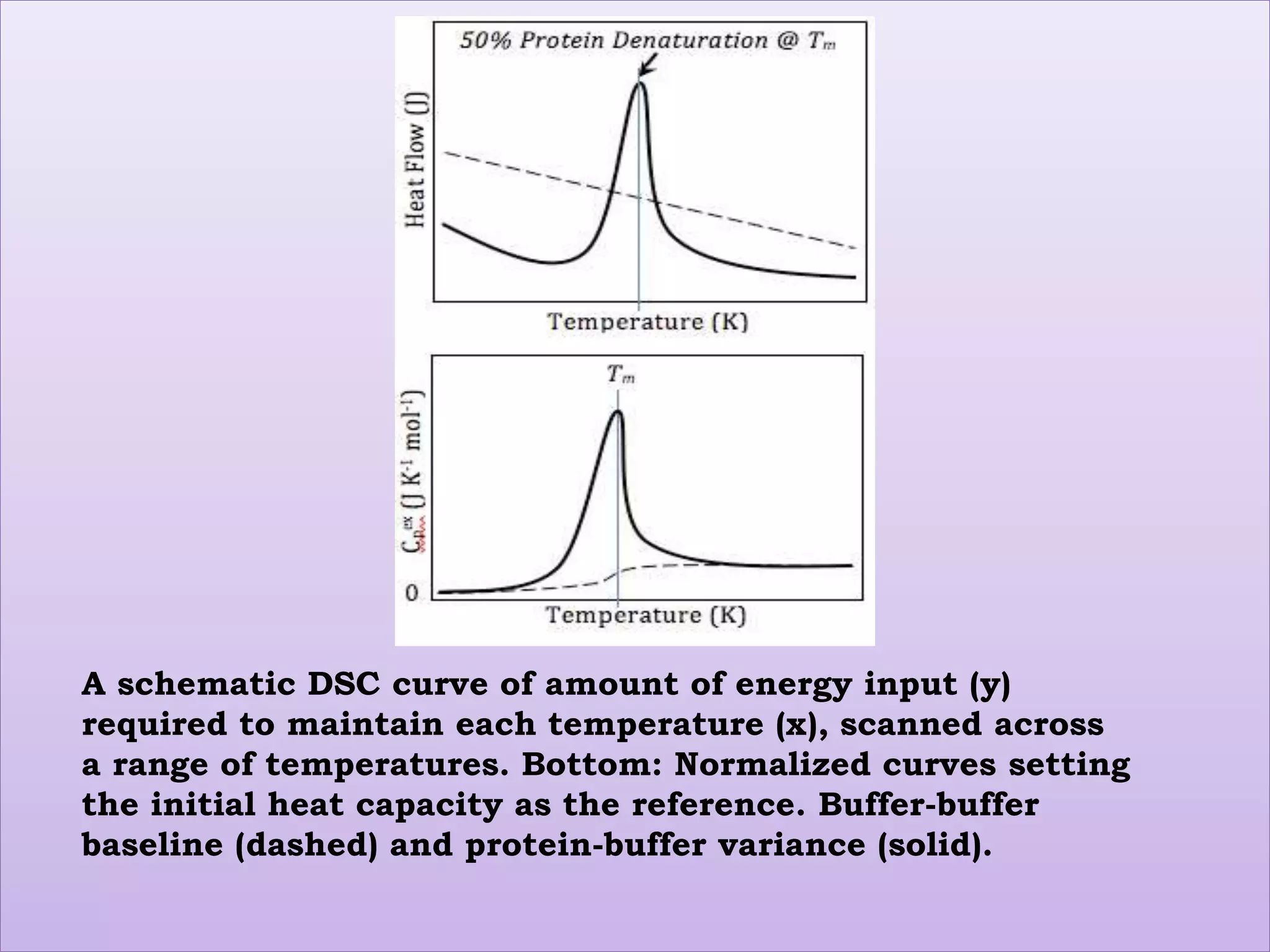
This document provides an overview of differential scanning calorimetry (DSC). DSC is a thermoanalytical technique that measures the heat flow into or out of a sample as it is heated or cooled. It can detect phase transitions like melting or glass transitions. The document discusses the principles, instrumentation, nature of DSC curves, factors affecting curves, and comparisons between DSC and differential thermal analysis.