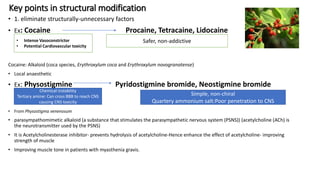
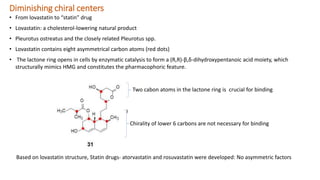
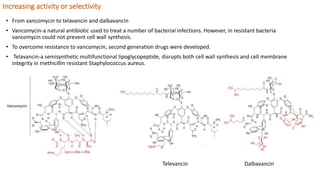
Structural modification of natural products is necessary to improve their properties for clinical use. Modifications aim to increase potency and selectivity, improve pharmacokinetic properties, eliminate toxicity, simplify structures while retaining activity, and generate patentable compounds. Key points of modification include eliminating unnecessary structural elements, introducing functional groups to boost binding, simplifying large complex structures, reducing chiral centers, increasing activity or selectivity, and improving physico-chemical properties. Examples provided demonstrate how natural products like cocaine, physostigmine, lovastatin, vancomycin, retinoic acid, and artemisinin were modified to develop safer, more effective drugs.