Embed presentation
Download to read offline





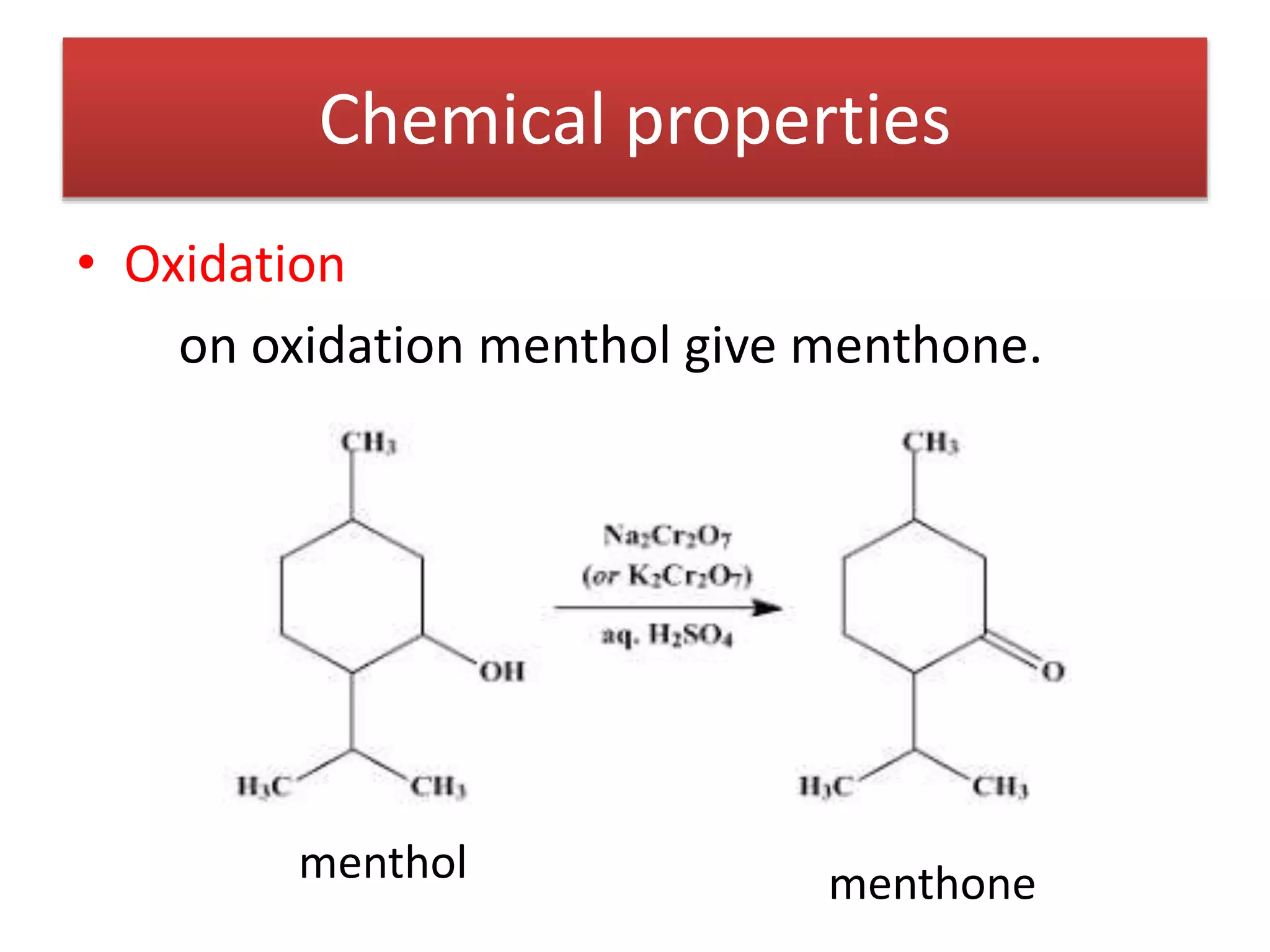

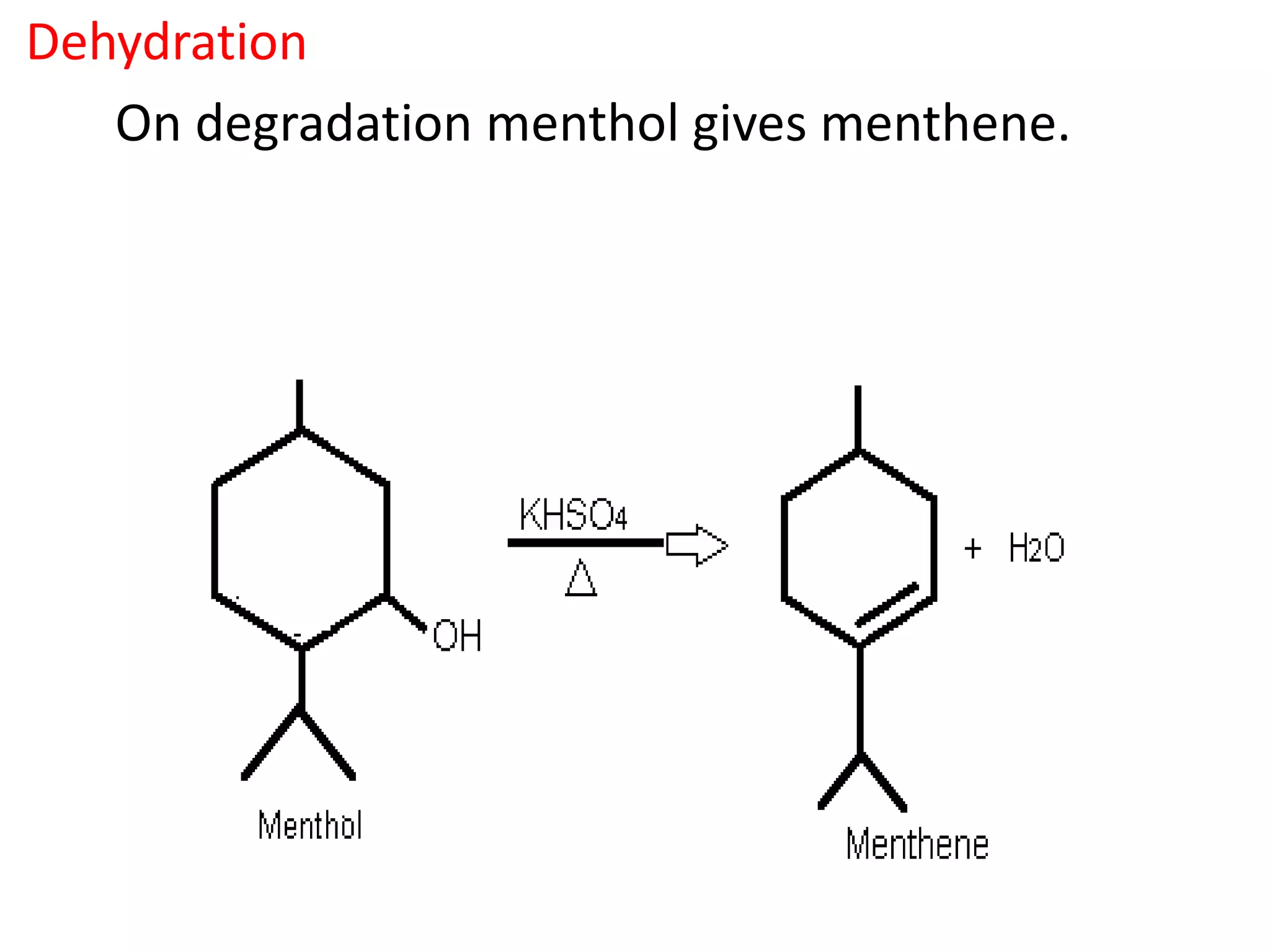
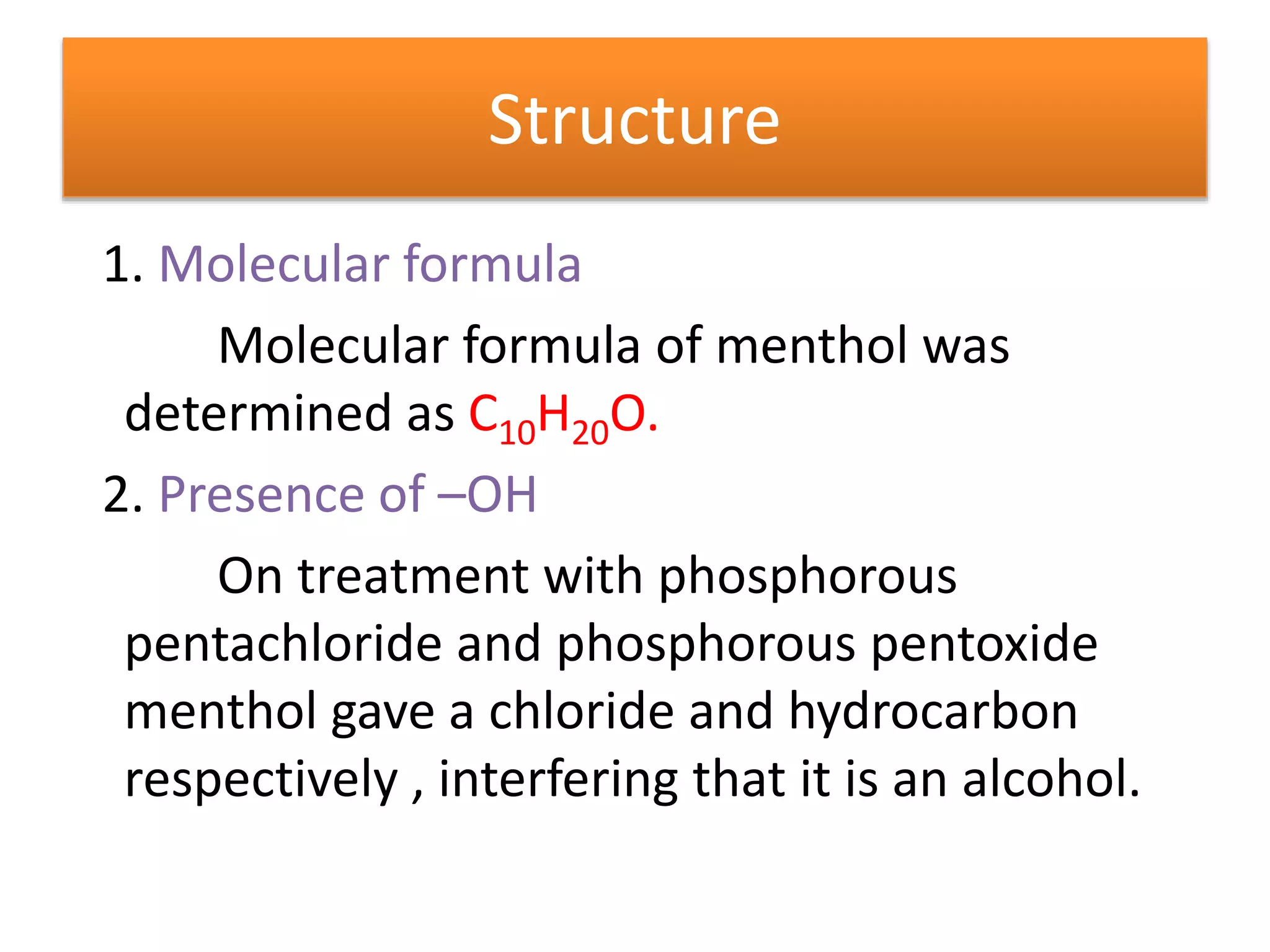


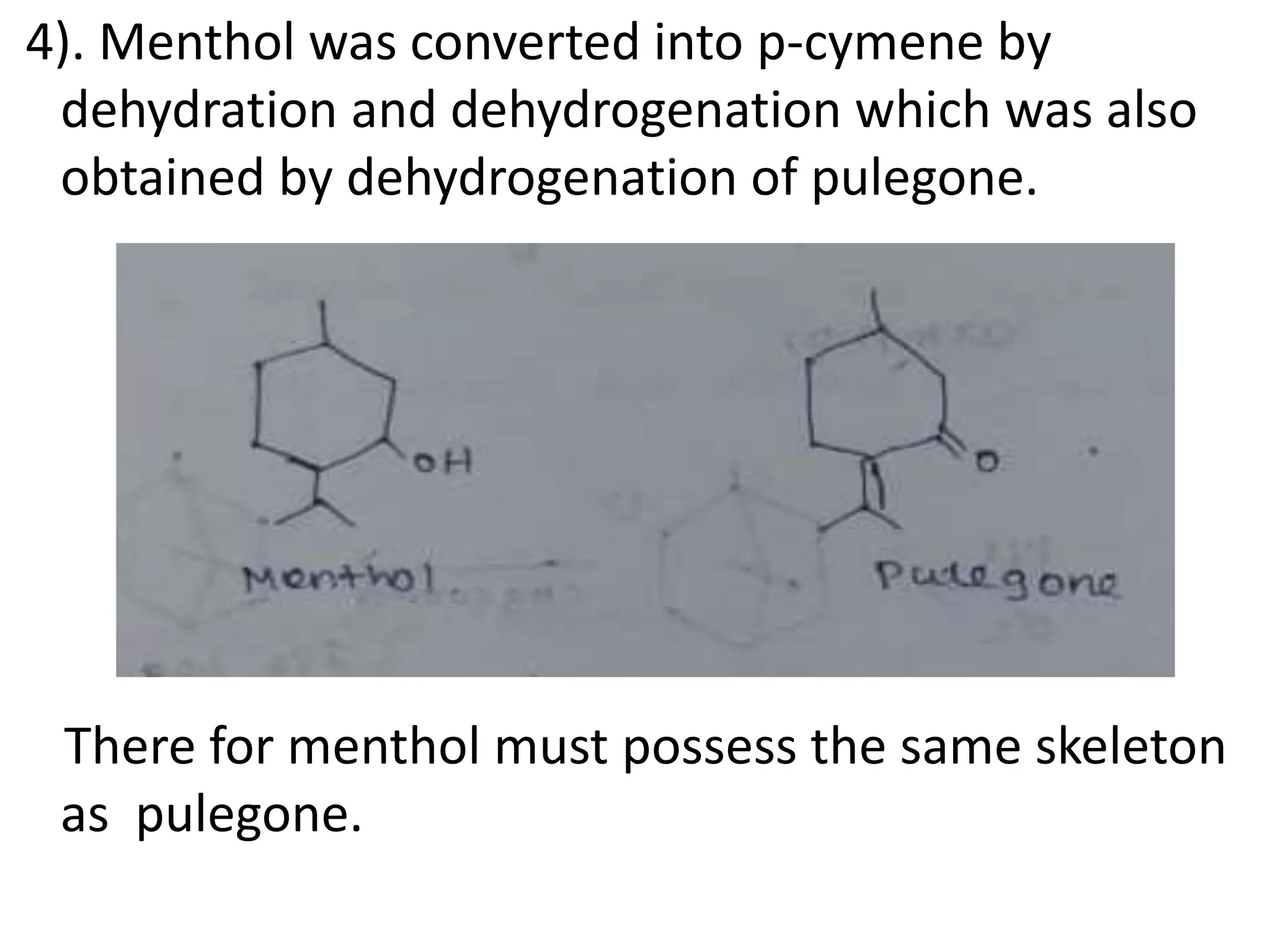

Menthol is obtained by reduction of pulegol. It is a colorless solid with a strong minty odor and cooling taste found in peppermint oil. Menthol undergoes oxidation to form menthone, reduction to form menthane, and dehydration to form menthene. Its molecular formula was determined to be C10H20O and it contains a secondary hydroxyl group, as shown by its conversion to a ketone when oxidized with chromic acid. Menthol has the same skeletal structure as pulegone, as menthol can be converted to p-cymene which is also obtained from dehydrogenation of pulegone.












