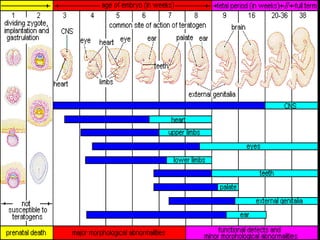
This document discusses drugs that can induce birth defects and the challenges of epidemiological research on this topic. It notes that 3-4% of live births experience major birth defects, and 40-90% of women consume at least one drug during pregnancy. Various drug classes like antibiotics, anticoagulants, NSAIDs, alcohol, and high-dose vitamin A are mentioned as potential teratogens. Methodological issues addressed include the rarity of specific birth defects requiring large sample sizes, recall bias in studies, and the need for cohort and case-control study designs. Solutions discussed involve different types of cohort studies and reviewing case reports to better understand adverse drug effects and design further research.