1. Audits, inspections, and monitoring are important quality assurance activities to ensure clinical trials are conducted properly and that human subjects and data are protected.
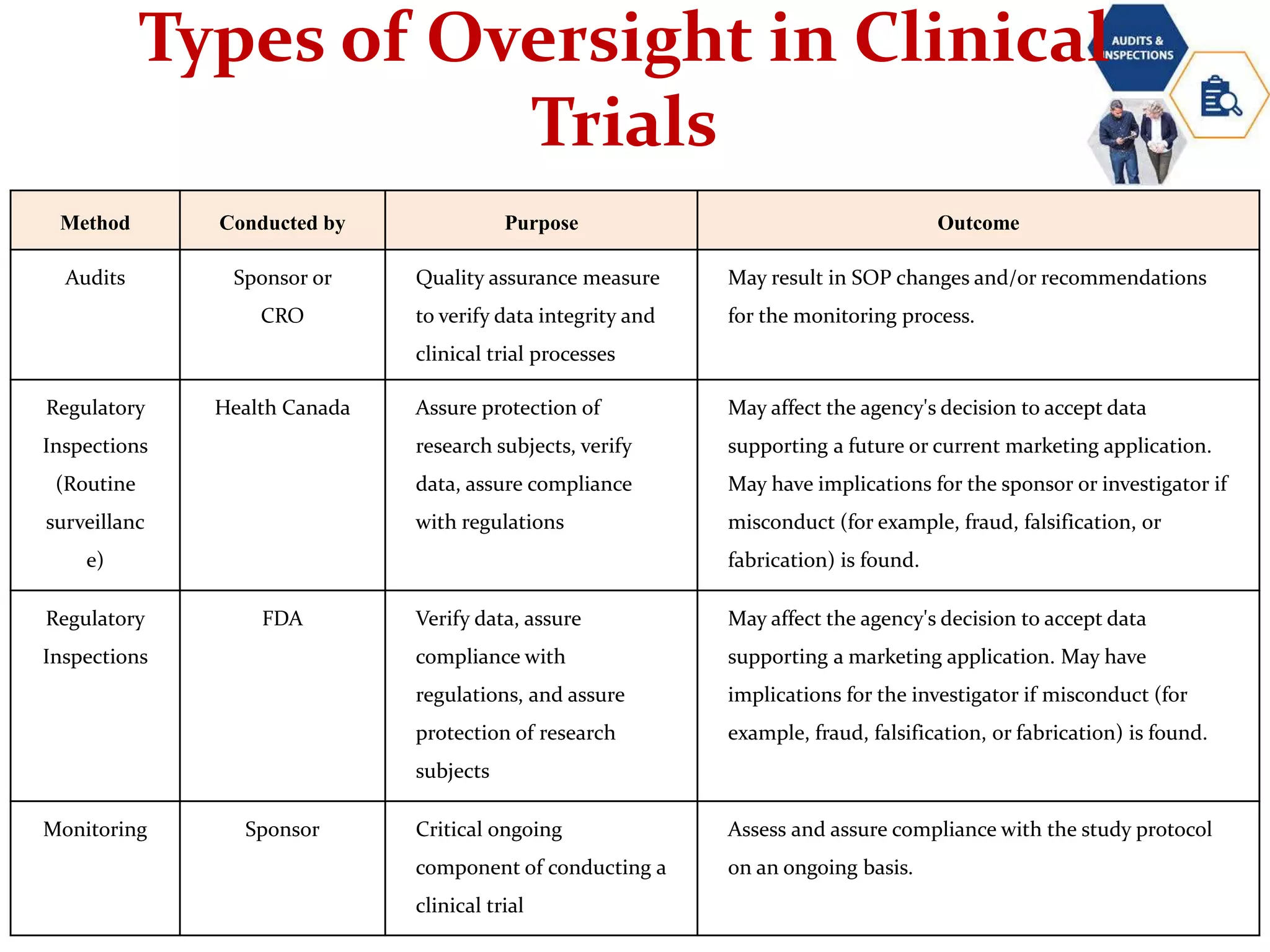
2. Audits examine trial activities and documents, inspections review documents and facilities for compliance, and monitoring oversees trial progress on an ongoing basis.
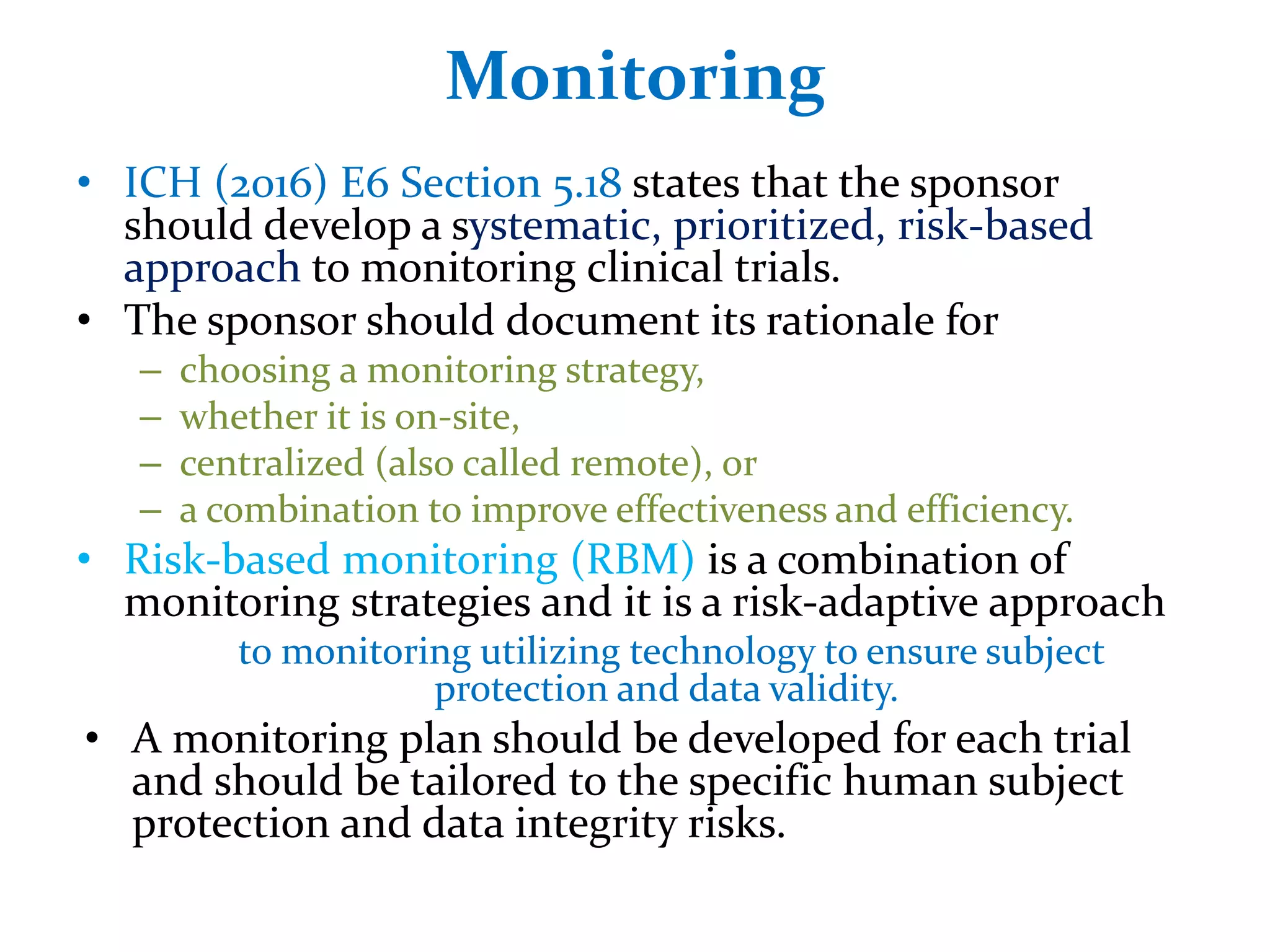
3. The main types of monitoring visits are pre-study visits to qualify sites, initiation visits to train staff, periodic visits to check compliance, and termination visits to close out the study.